Single-shot bevacizumab for cerebral radiation injury
- PMID: 33596839
- PMCID: PMC7888179
- DOI: 10.1186/s12883-021-02103-0
Single-shot bevacizumab for cerebral radiation injury
Abstract
Background: Cerebral radiation injury, including subacute radiation reactions and later stage radiation necrosis, is a severe side effect of brain tumor radiotherapy. A protocol of four infusions of the monoclonal antibody bevacizumab has been shown to be a highly effective treatment. However, bevacizumab is costly and can cause severe complications including thrombosis, bleeding and gastrointestinal perforations.
Methods: We performed a retrospective analysis of patients treated in our clinic for cerebral radiation injury who received only a singular treatment with bevacizumab. Single-shot was defined as a singular administration of bevacizumab without a second administration during an interval of at least 6 weeks.
Results: We identified 11 patients who had received a singular administration of bevacizumab to treat cerebral radiation injury. Prior radiation had been administered to treat gliomas (ten patients) or breast cancer brain metastases (one patient). 9 of 10 patients with available MRIs showed a marked reduction of edema at first follow-up. Discontinuation of Dexamethasone was possible in 6 patients and a significant dose reduction could be achieved in all other patients. One patient developed pulmonary artery embolism 2 months after bevacizumab administration. The median time to treatment failure of any cause was 3 months.
Conclusions: Single-shot bevacizumab therefore has meaningful activity in cerebral radiation injury, but durable control is rarely achieved. In patients where a complete protocol of four infusions with bevacizumab is not feasible due to medical contraindications or lack of reimbursement, single-shot bevacizumab treatment may be considered.
Keywords: Bevacizumab; Dexamethasone; Edema; Radiation necrosis; Side effect.
Conflict of interest statement
J.P.S. has received honoraria for lectures or advisory board participation, consulting or travel grants from Abbvie, Roche, Boehringer, Bristol-Myers Squibb, Medac, Mundipharma and UCB. M.W.R. has received a grant from UCB. The other authors report no conflict of interest.
Figures

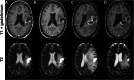

References
-
- Ellingson BM, Chung C, Pope WB, Boxerman JL, Kaufmann TJ. Pseudoprogression, radionecrosis, inflammation or true tumor progression? challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J Neurooncol. 2017;134 3:495–504. doi: 10.1007/s11060-017-2375-2. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

