Following cytotoxic nanoconjugates from injection to halting the cell cycle machinery and its therapeutic implications in oral cancer
- PMID: 33596850
- PMCID: PMC7890963
- DOI: 10.1186/s12885-021-07849-x
Following cytotoxic nanoconjugates from injection to halting the cell cycle machinery and its therapeutic implications in oral cancer
Abstract
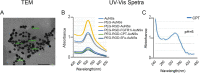
Background: The concept of personalized therapy has been proven to be a promising approach. A popular technique is to utilize gold nanoparticles (AuNPs) as drug delivery vectors for cytotoxic drugs and small molecule inhibitors to target and eradicate oral cancer cells in vitro and in vivo. Both drug and nanocarrier designs play important roles in the treatment efficacy. In our study, we standardized the nanosystem regarding NPs type, size, surface ligands and coverage percentage leaving only the drugs mode of action as the confounding variable. We propose that similarly constructed nanoparticles (NPs) can selectively leverage different conjugated drugs irrelevant to their original mode of action. If proven, AuNPs may have a secondary role beyond bypassing cancer cell membrane and delivering their loaded drugs.
Methods: We conjugated 5- fluorouracil (5Fu), camptothecin (CPT), and a fibroblast growth factor receptor1-inhibitor (FGFR1i) to gold nanospheres (AuNSs). We followed their trajectories in Syrian hamsters with chemically induced buccal carcinomas.
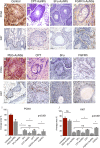
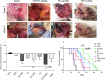
Results: Flow cytometry and cell cycle data shows that 5Fu- and CPT- induced a similar ratio of S-phase cell cycle arrest as nanoconjugates and in their free forms. On the other hand, FGFR1i-AuNSs induced significant sub-G1 cell population compared with its free form. Despite cell cycle dynamics variability, there was no significant difference in tumor cells' proliferation rate between CPT-, 5Fu- and FGFR1i- AuNSs treated groups. In our in vivo model, FGFR1i-AuNSs induced the highest tumor reduction rates followed by 5Fu- AuNSs. CPT-AuNSs induced significantly lower tumor reduction rates compared with the 5Fu- and FGFR1i- AuNSs despite showing similar proliferative rates in tumor cells.
Conclusions: Our data indicates that the cellular biological events do not predict the outcome seen in our in vivo model. Furthermore, our results suggest that AuNSs selectively enhance the therapeutic effect of small molecule inhibitors such as FGFR1i than potent anticancer drugs. Future studies are required to better understand the underlying mechanism.
Keywords: 5-flourouracil; Camptothecin; FGFR1 inhibitor, Oral cancer; Gold nanoparticles.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Function of gold nanoparticles in oral cancer beyond drug delivery: Implications in cell apoptosis.Oral Dis. 2021 Mar;27(2):251-265. doi: 10.1111/odi.13551. Epub 2020 Aug 6. Oral Dis. 2021. PMID: 32657515
-
XAV939: from a small inhibitor to a potent drug bioconjugate when delivered by gold nanoparticles.Bioconjug Chem. 2014 Feb 19;25(2):207-215. doi: 10.1021/bc400271x. Epub 2014 Jan 10. Bioconjug Chem. 2014. PMID: 24409808 Free PMC article.
-
Synergistic antitumor activity of the novel SN-38-incorporating polymeric micelles, NK012, combined with 5-fluorouracil in a mouse model of colorectal cancer, as compared with that of irinotecan plus 5-fluorouracil.Int J Cancer. 2008 May 1;122(9):2148-53. doi: 10.1002/ijc.23381. Int J Cancer. 2008. PMID: 18196580
-
Polymer decorated gold nanoparticles in nanomedicine conjugates.Adv Colloid Interface Sci. 2017 Nov;249:386-399. doi: 10.1016/j.cis.2017.01.007. Epub 2017 Feb 15. Adv Colloid Interface Sci. 2017. PMID: 28259207 Review.
-
Gold Nanoparticles for the Delivery of Cancer Therapeutics.Adv Cancer Res. 2018;139:163-184. doi: 10.1016/bs.acr.2018.05.001. Epub 2018 May 24. Adv Cancer Res. 2018. PMID: 29941104 Review.
Cited by
-
Novel tetrahydrocurcumin integrated mucoadhesive nanocomposite κ-carrageenan/xanthan gum sponges: a strategy for effective local treatment of oral cancerous and precancerous lesions.Drug Deliv. 2023 Dec;30(1):2254530. doi: 10.1080/10717544.2023.2254530. Drug Deliv. 2023. PMID: 37668361 Free PMC article.
-
Taiwan Green Propolis Nanoparticles Induce Antiproliferation and Apoptosis in Oral Cancer Cells.Biomedicines. 2025 Apr 9;13(4):921. doi: 10.3390/biomedicines13040921. Biomedicines. 2025. PMID: 40299536 Free PMC article.
-
Innovative nanoparticle strategies for treating oral cancers.Med Oncol. 2025 Apr 26;42(6):182. doi: 10.1007/s12032-025-02728-y. Med Oncol. 2025. PMID: 40285805 Review.
-
Nanoparticles as drug delivery systems in the treatment of oral squamous cell carcinoma: current status and recent progression.Front Pharmacol. 2023 May 24;14:1176422. doi: 10.3389/fphar.2023.1176422. eCollection 2023. Front Pharmacol. 2023. PMID: 37292147 Free PMC article. Review.
-
Mastoparan, a Peptide Toxin from Wasp Venom Conjugated Fluvastatin Nanocomplex for Suppression of Lung Cancer Cell Growth.Polymers (Basel). 2021 Dec 2;13(23):4225. doi: 10.3390/polym13234225. Polymers (Basel). 2021. PMID: 34883728 Free PMC article.
References
-
- Davis ME. Proceedings of the ASCO annual meeting 2014, Chicago, IL, USA, 30 may–3 June. 2014. Nanoparticle delivery platform for solid tumors.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

