Effect of prior cancer on survival outcomes for patients with advanced prostate cancer
- PMID: 33596896
- PMCID: PMC7891168
- DOI: 10.1186/s12894-021-00792-w
Effect of prior cancer on survival outcomes for patients with advanced prostate cancer
Abstract
Background: A history of prior cancer commonly results in exclusion from cancer clinical trials. However, whether a prior cancer history has an adversely impact on clinical outcomes for patients with advanced prostate cancer (APC) remains largely unknown. We therefore aimed to investigate the impact of prior cancer history on these patients.
Methods: We identified patients with advanced prostate cancer diagnosed from 2004 to 2010 in the Surveillance, Epidemiology, and End Results (SEER) database. Propensity score matching (PSM) was used to balance baseline characteristics. Kaplan-Meier method and the Cox proportional hazard model were utilized for survival analysis.
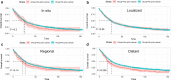
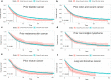
Results: A total of 19,772 eligible APC patients were included, of whom 887 (4.5 %) had a history of prior cancer. Urinary bladder (19 %), colon and cecum (16 %), melanoma of the skin (9 %) malignancies, and non-hodgkin lymphoma (9 %) were the most common types of prior cancer. Patients with a history of prior cancer had slightly inferior overall survival (OS) (AHR = 1.13; 95 % CI [1.02-1.26]; P = 0.017) as compared with that of patients without a prior cancer diagnosis. Subgroup analysis further indicated that a history of prior cancer didn't adversely impact patients' clinical outcomes, except in patients with a prior cancer diagnosed within 2 years, at advanced stage, or originating from specific sites, including bladder, colon and cecum, or lung and bronchus, or prior chronic lymphocytic leukemia.
Conclusions: A large proportion of APC patients with a prior cancer history had non-inferior survival to that of patients without a prior cancer diagnosis. These patients may be candidates for relevant cancer trials.
Keywords: Advanced prostate cancer; Prior cancer; SEER; Survival; Trial eligibility.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Wang YQ, Lv JW, Tang LL, Du XJ, Chen L, Li WF, Liu X, Guo Y, Lin AH, Mao YP, et al. Effect of prior cancer on trial eligibility and treatment outcomes in nasopharyngeal carcinoma: Implications for clinical trial accrual. Oral Oncol. 2019;90:23–9. doi: 10.1016/j.oraloncology.2019.01.023. - DOI - PubMed
-
- Filion M, Forget G, Brochu O, Provencher L, Desbiens C, Doyle C, Poirier B, DuRocher M, Camden S, Lemieux J. Eligibility criteria in randomized phase II and III adjuvant and neoadjuvant breast cancer trials: not a significant barrier to enrollment. Clin Trials. 2012;9(5):652–9. doi: 10.1177/1740774512456453. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

