Multi-modal imaging of high-risk ductal carcinoma in situ of the breast using C2Am: a targeted cell death imaging agent
- PMID: 33596961
- PMCID: PMC7891030
- DOI: 10.1186/s13058-021-01404-z
Multi-modal imaging of high-risk ductal carcinoma in situ of the breast using C2Am: a targeted cell death imaging agent
Erratum in
-
Correction to: Multi-modal imaging of high-risk ductal carcinoma in situ of the breast using C2Am: a targeted cell death imaging agent.Breast Cancer Res. 2021 Mar 15;23(1):32. doi: 10.1186/s13058-021-01414-x. Breast Cancer Res. 2021. PMID: 33722249 Free PMC article. No abstract available.
Abstract
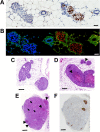
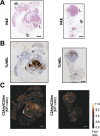
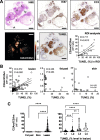
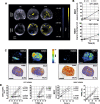
Background: Ductal carcinoma in situ (DCIS) is a non-invasive form of early breast cancer, with a poorly understood natural history of invasive transformation. Necrosis is a well-recognized adverse prognostic feature of DCIS, and non-invasive detection of its presence and spatial extent could provide information not obtainable by biopsy. We describe here imaging of the distribution and extent of comedo-type necrosis in a model of human DCIS using C2Am, an imaging agent that binds to the phosphatidylserine exposed by necrotic cells.
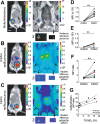
Methods: We used an established xenograft model of human DCIS that mimics the histopathological features of the disease. Planar near-infrared and optoacoustic imaging, using fluorescently labeled C2Am, were used to image non-invasively the presence and extent of lesion necrosis.
Results: C2Am showed specific and sensitive binding to necrotic areas in DCIS tissue, detectable both in vivo and ex vivo. The imaging signal generated in vivo using near-infrared (NIR) fluorescence imaging was up to 6-fold higher in DCIS lesions than in surrounding fat pad or skin tissue. There was a correlation between the C2Am NIR fluorescence (Pearson R = 0.783, P = 0.0125) and optoacoustic signals (R > 0.875, P < 0.022) in the DCIS lesions in vivo and the corresponding levels of cell death detected histologically.
Conclusions: C2Am is a targeted multi-modal imaging agent that could complement current anatomical imaging methods for detecting DCIS. Imaging the presence and spatial extent of necrosis may give better prognostic information than that obtained by biopsy alone.
Keywords: DCIS; Early detection; Multi-modal imaging; Necrosis; Optoacoustic.
Conflict of interest statement
C2Am is under a licensing agreement with Cambridge Enterprise and has been patented (US2011/0038798). Some of the authors of this study (AAN and KMB) are co-inventors on this patent. No other potential conflict of interest relevant to this article was reported.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

