Combined lymphocyte/monocyte count, D-dimer and iron status predict COVID-19 course and outcome in a long-term care facility
- PMID: 33596963
- PMCID: PMC7887565
- DOI: 10.1186/s12967-021-02744-2
Combined lymphocyte/monocyte count, D-dimer and iron status predict COVID-19 course and outcome in a long-term care facility
Abstract
Background: The Sars-CoV-2 can cause severe pneumonia with multiorgan disease; thus, the identification of clinical and laboratory predictors of the progression towards severe and fatal forms of this illness is needed. Here, we retrospectively evaluated and integrated laboratory parameters of 45 elderly subjects from a long-term care facility with Sars-CoV-2 outbreak and spread, to identify potential common patterns of systemic response able to better stratify patients' clinical course and outcome.
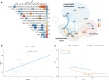
Methods: Baseline white blood cells, granulocytes', lymphocytes', and platelets' counts, hemoglobin, total iron, ferritin, D-dimer, and interleukin-6 concentration were used to generate a principal component analysis. Statistical analysis was performed by using R statistical package version 4.0.
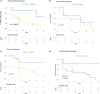
Results: We identified 3 laboratory patterns of response, renamed as low-risk, intermediate-risk, and high-risk, strongly associated with patients' survival (p < 0.01). D-dimer, iron status, lymphocyte/monocyte count represented the main markers discriminating high- and low-risk groups. Patients belonging to the high-risk group presented a significantly longer time to ferritin decrease (p: 0.047). Iron-to-ferritin-ratio (IFR) significantly segregated recovered and dead patients in the intermediate-risk group (p: 0.012).
Conclusions: Our data suggest that a combination of few laboratory parameters, i.e. iron status, D-dimer and lymphocyte/monocyte count at admission and during the hospital stay, can predict clinical progression in COVID-19.
Keywords: COVID-19; Clinical outcome; D-dimer; Iron; Long-term care facilities; Lymphocyte; Monocyte.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

