Belgian rare diseases plan in clinical pathology: identification of key biochemical diagnostic tests and establishment of reference laboratories and financing conditions
- PMID: 33596965
- PMCID: PMC7890854
- DOI: 10.1186/s13023-021-01728-1
Belgian rare diseases plan in clinical pathology: identification of key biochemical diagnostic tests and establishment of reference laboratories and financing conditions
Abstract
Background: One objective of the Belgian Rare Diseases plan is to improve patients' management using phenotypic tests and, more specifically, the access to those tests by identifying the biochemical analyses used for rare diseases, developing new financing conditions and establishing reference laboratories.
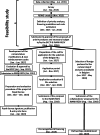
Methods: A feasibility study was performed from May 2015 until August 2016 in order to select the financeable biochemical analyses, and, among them, those that should be performed by reference laboratories. This selection was based on an inventory of analyses used for rare diseases and a survey addressed to the Belgian laboratories of clinical pathology (investigating the annual analytical costs, volumes, turnaround times and the tests unavailable in Belgium and outsourced abroad). A proposal of financeable analyses, financing modalities, reference laboratories' scope and budget estimation was developed and submitted to the Belgian healthcare authorities. After its approval in December 2016, the implementation phase took place from January 2017 until December 2019.
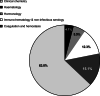
Results: In 2019, new reimbursement conditions have been published for 46 analyses and eighteen reference laboratories have been recognized. Collaborations have also been developed with 5 foreign laboratories in order to organize the outsourcing and financing of 9 analyses unavailable in Belgium.
Conclusions: In the context of clinical pathology and rare diseases, this initiative enabled to identify unreimbursed analyses and to meet the most crucial financial needs. It also contributed to improve patients' management by establishing Belgian reference laboratories and foreign referral laboratories for highly-specific analyses and a permanent surveillance, quality and financing framework for those tests.
Keywords: Clinical pathology; Expertise; Financing; Rare diseases; Reference laboratories; Reimbursement codes.
Conflict of interest statement
The RDWG members involved in the priority analyses selection work in some of the laboratories performing the analyses covered in this paper.
Figures


Similar articles
-
Exploring alternative financing models and early access schemes for orphan drugs: a Belgian case study.Orphanet J Rare Dis. 2022 Dec 9;17(1):429. doi: 10.1186/s13023-022-02571-8. Orphanet J Rare Dis. 2022. PMID: 36494733 Free PMC article.
-
Belgian Recommendations for Analytical Verification and Validation of Immunohistochemical Tests in Laboratories of Anatomic Pathology.Appl Immunohistochem Mol Morphol. 2024 Jan 1;32(1):1-16. doi: 10.1097/PAI.0000000000001165. Epub 2023 Oct 17. Appl Immunohistochem Mol Morphol. 2024. PMID: 38054253 Free PMC article.
-
Belgian guidelines for budget impact analyses.Acta Clin Belg. 2015 Jun;70(3):175-80. doi: 10.1179/2295333714Y.0000000118. Epub 2015 Jan 11. Acta Clin Belg. 2015. PMID: 25579611
-
Reference centres for adults with rare and complex cancers - Policy recommendations to improve the organisation of care in Belgium.Rev Epidemiol Sante Publique. 2016 Feb;64(1):1-6. doi: 10.1016/j.respe.2015.11.006. Epub 2015 Dec 30. Rev Epidemiol Sante Publique. 2016. PMID: 26745998 Review.
-
The Importance of Establishing Reference Intervals - is it still a Current Problem for Laboratory and Doctors?Clin Lab. 2020 Aug 1;66(8). doi: 10.7754/Clin.Lab.2020.191120. Clin Lab. 2020. PMID: 32776738 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

