Conditioned medium from primary cytotrophoblasts, primary placenta-derived mesenchymal stem cells, or sub-cultured placental tissue promoted HUVEC angiogenesis in vitro
- PMID: 33596987
- PMCID: PMC7890636
- DOI: 10.1186/s13287-021-02192-1
Conditioned medium from primary cytotrophoblasts, primary placenta-derived mesenchymal stem cells, or sub-cultured placental tissue promoted HUVEC angiogenesis in vitro
Abstract
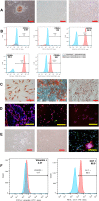
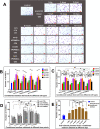
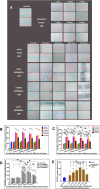
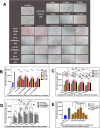
Background: As a large capillary network, the human placenta plays an important role throughout pregnancy. Placental vascular development is complex and delicate and involves many types of placental cells, such as trophoblasts, and mesenchymal stem cells. There has been no systematic, comparative study on the roles of these two groups of placental cells and the whole placental tissue in the placental angiogenesis. In this study, primary cytotrophoblasts (CTBs) from early pregnancy and primary human placenta-derived mesenchymal stem cells (hPDMSCs) from different stages of pregnancy were selected as the cell research objects, and full-term placental tissue was selected as the tissue research object to detect the effects of their conditioned medium (CM) on human umbilical vein endothelial cell (HUVEC) angiogenesis.
Methods: We successfully isolated primary hPDMSCs and CTBs, collected CM from these placental cells and sub-cultured placental tissue, and then evaluated the effects of the CM on a series of angiogenic processes in HUVECs in vitro. Furthermore, we measured the levels of angiogenic factors in the CM of placental cells or tissue by an angiogenesis antibody array.
Results: The results showed that not only placental cells but also sub-cultured placental tissue, to some extent, promoted HUVEC angiogenesis in vitro by promoting proliferation, adhesion, migration, invasion, and tube formation. We also found that primary placental cells in early pregnancy, whether CTBs or hPDMSCs, played more significant roles than those in full-term pregnancy. Placental cell-derived CM collected at 24 h or 48 h had the best effect, and sub-cultured placental tissue-derived CM collected at 7 days had the best effect among all the different time points. The semiquantitative angiogenesis antibody array showed that 18 of the 43 angiogenic factors had obvious spots in placental cell-derived CM or sub-cultured placental tissue-derived CM, and the levels of 5 factors (including CXCL-5, GRO, IL-6, IL-8, and MCP-1) were the highest in sub-cultured placental tissue-derived CM.
Conclusions: CM obtained from placental cells (primary CTBs or hPDMSCs) or sub-cultured placental tissue contained proangiogenic factors and promoted HUVEC angiogenesis in vitro. Therefore, our research is helpful to better understand placental angiogenesis regulation and provides theoretical support for the clinical application of placental components, especially sub-cultured placental tissue-derived CM, in vascular tissue engineering and clinical treatments.
Keywords: Angiogenesis; Conditioned medium; HUVECs; Placental tissue; Primary cytotrophoblasts; Primary human placenta-derived mesenchymal stem cells.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Wang Y, Zhao S. Vascular biology of the placenta. San Rafael: Morgan & Claypool Life Sciences; 2010. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

