Efficacy of metformin and fermentable fiber combination therapy in adolescents with severe obesity and insulin resistance: study protocol for a double-blind randomized controlled trial
- PMID: 33596993
- PMCID: PMC7890810
- DOI: 10.1186/s13063-021-05060-8
Efficacy of metformin and fermentable fiber combination therapy in adolescents with severe obesity and insulin resistance: study protocol for a double-blind randomized controlled trial
Abstract
Background: Accumulating evidence suggests that the metabolic effects of metformin and fermentable fibers are mediated, in part, through diverging or overlapping effects on the composition and metabolic functions of the gut microbiome. Pre-clinical animal models have established that the addition of fiber to metformin monotherapy improves glucose tolerance. However, possible synergistic effects of combination therapy (metformin plus fiber) have not been investigated in humans. Moreover, the underlying mechanisms of synergy have yet to be elucidated. The aim of this study is to compare in adolescents with obesity the metabolic effects of metformin and fermentable fibers in combination with those of metformin or fiber alone. We will also determine if therapeutic responses correlate with compositional and functional features of the gut microbiome.
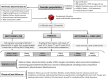
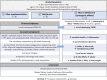
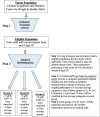
Methods: This is a parallel three-armed, double-blinded, randomized controlled trial. Adolescents (aged 12-18 years) with obesity, insulin resistance (IR), and a family history of type 2 diabetes mellitus (T2DM) will receive either metformin (850 mg p.o. twice/day), fermentable fibers (35 g/day), or a combination of metformin plus fiber for 12 months. Participants will be seen at baseline, 3, 6, and 12 months, with a phone follow-up at 1 and 9 months. Primary and secondary outcomes will be assessed at baseline, 6, and 12 months. The primary outcome is change in IR estimated by homeostatic model assessment of IR; key secondary outcomes include changes in the Matsuda index, oral disposition index, body mass index z-score, and fat mass to fat-free mass ratio. To gain mechanistic insight, endpoints that reflect host-microbiota interactions will also be assessed: obesity-related immune, metabolic, and satiety markers; humoral metabolites; and fecal microbiota composition, short-chain fatty acids, and bile acids.
Discussion: This study will compare the potential metabolic benefits of fiber with those of metformin in adolescents with obesity, determine if metformin and fiber act synergistically to improve IR, and elucidate whether the metabolic benefits of metformin and fiber associate with changes in fecal microbiota composition and the output of health-related metabolites. This study will provide insight into the potential role of the gut microbiome as a target for enhancing the therapeutic efficacy of emerging treatments for T2DM prevention.
Trial registration: ClinicalTrials.gov NCT04578652 . Registered on 8 October 2020.
Keywords: Adolescents; Diabetes; Dietary fiber; Gut microbiome; Insulin resistance; Metformin; Obesity.
Figures
References
-
- Group TS. Zeitler P, Epstein L, Grey M, Hirst K, Kaufman F, et al. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8(2):74–87. doi: 10.1111/j.1399-5448.2007.00237.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

