Nanobody Nb6 fused with porcine IgG Fc as the delivering tag to inhibit porcine reproductive and respiratory syndrome virus replication in porcine alveolar macrophages
- PMID: 33596995
- PMCID: PMC7887809
- DOI: 10.1186/s13567-020-00868-9
Nanobody Nb6 fused with porcine IgG Fc as the delivering tag to inhibit porcine reproductive and respiratory syndrome virus replication in porcine alveolar macrophages
Abstract
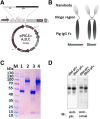
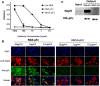
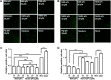
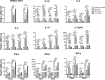
Porcine reproductive and respiratory syndrome virus (PRRSV) is a highly contagious virus that has led to enormous economic loss worldwide because of ineffective prevention and treatment. In view of their minimized size, high target specificity and affinity, nanobodies have been extensively investigated as diagnostic tools and treatments of many diseases. Previously, a PRRSV Nsp9-specific nanobody (Nb6) was identified as a PRRSV replication inhibitor. When it was fused with cell-penetrating peptide (CPP) TAT, Nb6-TAT could enter the cells for PRRSV suppression. However, delivery of molecules by CPP lack cell specificity and have a short duration of action. PRRSV has a tropism for monocyte/macrophage lineage, which expresses high levels of Fcγ receptors. Herein, we designed a nanobody containing porcine IgG Fc (Fcγ) to inhibit PRRSV replication in PRRSV permissive cells. Fcγ fused Nb6 chimeric antibody (Nb6-pFc) was assembled into a dimer with interchain disulfide bonds and expressed in a Pichia pastoris system. The results show that Nb6-pFc exhibits a well-binding ability to recombinant Nsp9 or PRRSV-encoded Nsp9 and that FcγR-mediated endocytosis of Nb6-pFc into porcine alveolar macrophages (PAM) was in a dose-dependent manner. Nb6-pFc can inhibit PRRSV infection efficiently not only by binding with Nsp9 but also by upregulating proinflammatory cytokine production in PAM. Together, this study proposes the design of a porcine IgG Fc-fused nanobody that can enter PRRSV susceptible PAM via FcγR-mediated endocytosis and inhibit PRRSV replication. This research reveals that nanobody-Fcγ chimeric antibodies might be effective for the control and prevention of monocyte/macrophage lineage susceptible pathogeneses.
Keywords: PRRSV; antiviral agents; nanobody; nanobody-pFc; permissive cell targeting.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

