Gut microbiota of patients with different subtypes of gastric cancer and gastrointestinal stromal tumors
- PMID: 33596997
- PMCID: PMC7888145
- DOI: 10.1186/s13099-021-00403-x
Gut microbiota of patients with different subtypes of gastric cancer and gastrointestinal stromal tumors
Abstract
Background: Gastric adenocarcinoma is associated with H. pylori infection and inflammation that can result in the dysbiosis of gastric microbiota. The association of intestinal microbiota with gastric adenocarcinoma subtypes or with gastric gastrointestinal stromal tumors (GIST) is however not well known. Therefore, we performed 16S rRNA gene sequencing on DNA isolated from stool samples of Finnish patients and controls to study differences in microbiota among different histological subtypes of gastric adenocarcinoma, gastric GIST and healthy controls.
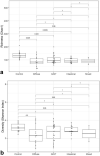
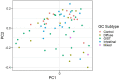
Results: We found that gut microbiota alpha diversity was lowest in diffuse adenocarcinoma patients, followed by intestinal type and GIST patients, although the differences were not significant compared to controls. Beta-diversity analysis however showed significant differences in microbiota composition for all subtypes compared to controls. Significantly higher abundance of Enterobacteriaceae was observed in both adenocarcinoma subtypes, whereas lower abundance of Bifidobacteriaceae was seen only in diffuse adenocarcinoma and of Oscillibacter in intestinal adenocarcinoma. Both GIST and adenocarcinoma patients had higher abundance of Enterobacteriaceae and lower abundance of Lactobacillaceae and Oscillibacter while lower abundance of Lachnoclostridium, Bifidobacterium, Parabacteroides and Barnesiella was seen only in the adenocarcinoma patients.
Conclusions: Our analysis shows association of higher Enterobacteriaceae abundance with all types of gastric tumors. Therefore it could be potentially useful as a marker of gastric malignancies. Lower gut microbiota diversity might be indicative of poorly differentiated, invasive, advanced or aggressive tumors and could possibly be a prognostic marker for gastric tumors.
Keywords: Diffuse gastric adenocarcinoma; GIST; Gut microbiota; Intestinal gastric adenocarcinoma.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Wang X, Wei M, Sun Z. An association study of histological types of gastric carcinoma with Helicobacter pylori infection. Cell Biochem Biophys. 2014;70:283–287. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

