Differential SOD2 and GSTZ1 profiles contribute to contrasting dental pulp stem cell susceptibilities to oxidative damage and premature senescence
- PMID: 33596998
- PMCID: PMC7890809
- DOI: 10.1186/s13287-021-02209-9
Differential SOD2 and GSTZ1 profiles contribute to contrasting dental pulp stem cell susceptibilities to oxidative damage and premature senescence
Abstract
Background: Dental pulp stem cells (DPSCs) are increasingly being advocated as viable cell sources for regenerative medicine-based therapies. However, significant heterogeneity in DPSC expansion and multi-potency capabilities are well-established, attributed to contrasting telomere profiles and susceptibilities to replicative senescence. As DPSCs possess negligible human telomerase (hTERT) expression, we examined whether intrinsic differences in the susceptibilities of DPSC sub-populations to oxidative stress-induced biomolecular damage and premature senescence further contributed to this heterogeneity, via differential enzymic antioxidant capabilities between DPSCs.
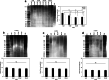
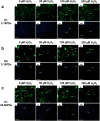
Methods: DPSCs were isolated from human third molars by differential fibronectin adhesion, and positive mesenchymal (CD73/CD90/CD105) and negative hematopoietic (CD45) stem cell marker expression confirmed. Isolated sub-populations were expanded in H2O2 (0-200 μM) and established as high or low proliferative DPSCs, based on population doublings (PDs) and senescence (telomere lengths, SA-β-galactosidase, p53/p16INK4a/p21waf1/hTERT) marker detection. The impact of DPSC expansion on mesenchymal, embryonic, and neural crest marker expression was assessed, as were the susceptibilities of high and low proliferative DPSCs to oxidative DNA and protein damage by immunocytochemistry. Expression profiles for superoxide dismutases (SODs), catalase, and glutathione-related antioxidants were further compared between DPSC sub-populations by qRT-PCR, Western blotting and activity assays.
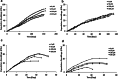
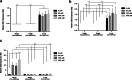
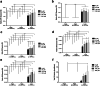
Results: High proliferative DPSCs underwent > 80PDs in culture and resisted H2O2-induced senescence (50-76PDs). In contrast, low proliferative sub-populations exhibited accelerated senescence (4-32PDs), even in untreated controls (11-34PDs). While telomere lengths were largely unaffected, certain stem cell marker expression declined with H2O2 treatment and expansion. Elevated senescence susceptibilities in low proliferative DPSC (2-10PDs) were accompanied by increased oxidative damage, absent in high proliferative DPSCs until 45-60PDs. Increased SOD2/glutathione S-transferase ζ1 (GSTZ1) expression and SOD activities were identified in high proliferative DPSCs (10-25PDs), which declined during expansion. Low proliferative DPSCs (2-10PDs) exhibited inferior SOD, catalase and glutathione-related antioxidant expression/activities.
Conclusions: Significant variations exist in the susceptibilities of DPSC sub-populations to oxidative damage and premature senescence, contributed to by differential SOD2 and GSTZ1 profiles which maintain senescence-resistance/stemness properties in high proliferative DPSCs. Identification of superior antioxidant properties in high proliferative DPSCs enhances our understanding of DPSC biology and senescence, which may be exploited for selective sub-population screening/isolation from dental pulp tissues for regenerative medicine-based applications.
Keywords: Dental pulp stem cells; GSTZ1; Heterogeneity; Oxidative damage; Oxidative stress; Premature senescence; SOD2.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





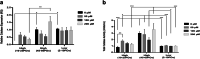

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

