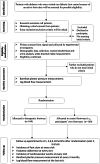
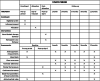
Effects of novel diabetic therapeutic footwear on preventing ulcer recurrence in patients with a history of diabetic foot ulceration: study protocol for an open-label, randomized, controlled trial
- PMID: 33597005
- PMCID: PMC7890642
- DOI: 10.1186/s13063-021-05098-8
Effects of novel diabetic therapeutic footwear on preventing ulcer recurrence in patients with a history of diabetic foot ulceration: study protocol for an open-label, randomized, controlled trial
Abstract
Background: Recurrence after the healing of a foot ulcer is very common among patients with diabetes mellitus. Novel diabetic therapeutic footwear consisted of merino wool, vibration chip, and orthopedic insoles is designed to influence multifaceted mechanisms of foot ulcer occurrence. The aim of this study is to examine the effect of the optimally designed therapeutic footwear on preventing ulcer recurrence in patients with a history of diabetic foot ulcers (DFU).
Methods/design: The trial is designed as a two arms, parallel-group, open-label randomized controlled intervention study. The Log-rank Test was used for calculating sample size based on the latest national multicenter survey data of DFU in China. Three hundred and twenty participants will be recruited from the Diabetic Foot Care Center, West China Hospital, Sichuan University. Adults with diabetic peripheral neuropathy, healed foot ulceration in the 3 months prior to randomization, and aged ≥18 years, will be recruited. Participants will be randomized to receive novel diabetic therapeutic footwear (n = 160) or their own footwear (n = 160). The primary outcome will be the incidence of ulcer recurrence. The secondary outcome will be measurements of barefoot dynamic plantar pressures, the influence of footwear adherence on ulcer recurrence, and the incidence of cardiovascular events. Assessment visits and data collection will be obtained at baseline, 1, 3, 6, 9, and 12 months. The intention-to-treat principle will be applied. A cox regression model will be used to calculate the hazard ratio for the incidence of ulcer recurrence. The change of barefoot dynamic plantar pressures will be assessed using repeated measures ANOVA. The study protocol has been approved by the Ethics Committee of The Biomedical Research Ethics Committee of West China Hospital, Sichuan University (Reference No. 2019(96)).
Discussion: This clinical trial will give information on the ability of novel diabetic footwear on preventing ulcer recurrence in patients with a history of diabetic foot ulceration. If the optimally designed therapeutic footwear does work well, the findings will contribute to the development of innovative treatment devices for preventing foot ulcer recurrence in high-risk patients.
Trial registration: Chinese Clinical Trial Registry ChiCTR1900025538 . Registered on 31 August 2019.
Keywords: Adherence to treatment; Diabetic foot ulcers; Diabetic therapeutic footwear; Randomized controlled trial; Ulcer recurrence.
Conflict of interest statement
JHC received honoraria from Korean military, le Mouton Shoes (KOREA), Ounce shoes (CHINA), Hanbee Shoes (KOREA), is the founder of DAJIM INC KOREA and serves as the Chief Knowledge Officer at DAJIM INC KOREA. YC received honoraria from Ounce shoes (CHINA), is the founder of Ounce and serves as director for Foot Health Research Center at Beijing Ounce Health Technology Co., Ltd. YZL received honoraria from Ounce shoes (CHINA), is the founder of Ounce and serves as director for Research &Development Center at Beijing Ounce Health Technology Co., Ltd. YG, CW, DWC, HH, LHC, GJL, SL, ML, and XWR declare that they have no conflict of interest.
Figures
Similar articles
-
An integrated personalized assistive devices approach to reduce the risk of foot ulcer recurrence in diabetes (DIASSIST): study protocol for a multicenter randomized controlled trial.Trials. 2023 Oct 12;24(1):663. doi: 10.1186/s13063-023-07635-z. Trials. 2023. PMID: 37828618 Free PMC article.
-
Clinical efficacy of therapeutic footwear with a rigid rocker sole in the prevention of recurrence in patients with diabetes mellitus and diabetic polineuropathy: A randomized clinical trial.PLoS One. 2019 Jul 11;14(7):e0219537. doi: 10.1371/journal.pone.0219537. eCollection 2019. PLoS One. 2019. PMID: 31295292 Free PMC article. Clinical Trial.
-
Footwear and insole design parameters to prevent occurrence and recurrence of neuropathic plantar forefoot ulcers in patients with diabetes: a series of N-of-1 trial study protocol.Trials. 2022 Dec 16;23(1):1017. doi: 10.1186/s13063-022-06968-5. Trials. 2022. PMID: 36527100 Free PMC article.
-
Footwear and insole design features that reduce neuropathic plantar forefoot ulcer risk in people with diabetes: a systematic literature review.J Foot Ankle Res. 2020 Jun 4;13(1):30. doi: 10.1186/s13047-020-00400-4. J Foot Ankle Res. 2020. PMID: 32498719 Free PMC article.
-
An integrative review of therapeutic footwear for neuropathic foot due to diabetes mellitus.Diabetes Metab Syndr. 2019 Mar-Apr;13(2):913-923. doi: 10.1016/j.dsx.2018.12.011. Epub 2018 Dec 21. Diabetes Metab Syndr. 2019. PMID: 31336545 Review.
Cited by
-
Orthotic approach to prevention and management of diabetic foot: A narrative review.World J Diabetes. 2022 Nov 15;13(11):912-920. doi: 10.4239/wjd.v13.i11.912. World J Diabetes. 2022. PMID: 36437865 Free PMC article. Review.
-
Diabetic foot prevention, assessment, and management using innovative smart wearable technology: a systematic review.J Neuroeng Rehabil. 2025 Jul 18;22(1):168. doi: 10.1186/s12984-025-01695-9. J Neuroeng Rehabil. 2025. PMID: 40682082 Free PMC article. Review.
-
The Design of Individual Orthopedic Insoles for the Patients with Diabetic Foot Using Integral Curves to Describe the Plantar Over-Pressure Areas.Comput Math Methods Med. 2021 Aug 6;2021:9061241. doi: 10.1155/2021/9061241. eCollection 2021. Comput Math Methods Med. 2021. PMID: 34413899 Free PMC article.
-
Custom-Molded Offloading Footwear Effectively Prevents Recurrence and Amputation, and Lowers Mortality Rates in High-Risk Diabetic Foot Patients: A Multicenter, Prospective Observational Study.Diabetes Metab Syndr Obes. 2022 Jan 10;15:103-109. doi: 10.2147/DMSO.S341364. eCollection 2022. Diabetes Metab Syndr Obes. 2022. PMID: 35046681 Free PMC article.
References
-
- Armstrong DG, Fisher TK, Lepow B, White ML, Mills JL, Fitridge R, et al. Pathophysiology and principles of management of the diabetic foot. In: Fitridge R, Thompson M, et al., editors. Mechanisms of vascular disease: a reference book for vascular specialists. Adelaide: University of Adelaide Press; 2011. pp. 475–496. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical