CERTL reduces C16 ceramide, amyloid-β levels, and inflammation in a model of Alzheimer's disease
- PMID: 33597019
- PMCID: PMC7890977
- DOI: 10.1186/s13195-021-00780-0
CERTL reduces C16 ceramide, amyloid-β levels, and inflammation in a model of Alzheimer's disease
Abstract
Background: Dysregulation of ceramide and sphingomyelin levels have been suggested to contribute to the pathogenesis of Alzheimer's disease (AD). Ceramide transfer proteins (CERTs) are ceramide carriers which are crucial for ceramide and sphingomyelin balance in cells. Extracellular forms of CERTs co-localize with amyloid-β (Aβ) plaques in AD brains. To date, the significance of these observations for the pathophysiology of AD remains uncertain.
Methods: A plasmid expressing CERTL, the long isoform of CERTs, was used to study the interaction of CERTL with amyloid precursor protein (APP) by co-immunoprecipitation and immunofluorescence in HEK cells. The recombinant CERTL protein was employed to study interaction of CERTL with amyloid-β (Aβ), Aβ aggregation process in presence of CERTL, and the resulting changes in Aβ toxicity in neuroblastoma cells. CERTL was overexpressed in neurons by adeno-associated virus (AAV) in a mouse model of familial AD (5xFAD). Ten weeks after transduction, animals were challenged with behavior tests for memory, anxiety, and locomotion. At week 12, brains were investigated for sphingolipid levels by mass spectrometry, plaques, and neuroinflammation by immunohistochemistry, gene expression, and/or immunoassay.
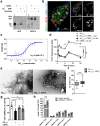
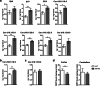
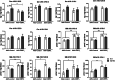
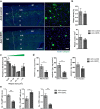
Results: Here, we report that CERTL binds to APP, modifies Aβ aggregation, and reduces Aβ neurotoxicity in vitro. Furthermore, we show that intracortical injection of AAV, mediating the expression of CERTL, decreases levels of ceramide d18:1/16:0 and increases sphingomyelin levels in the brain of male 5xFAD mice. CERTL in vivo over-expression has a mild effect on animal locomotion, decreases Aβ formation, and modulates microglia by decreasing their pro-inflammatory phenotype.
Conclusion: Our results demonstrate a crucial role of CERTL in regulating ceramide levels in the brain, in amyloid plaque formation and neuroinflammation, thereby opening research avenues for therapeutic targets of AD and other neurodegenerative diseases.
Keywords: 5xFAD; Adeno-associated virus (AAV); Alzheimer’s disease (AD); Amyloid-β plaques; Ceramide; Ceramide transporter protein (CERT); Microglia; Neuroinflammation; Sphingomyelin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Crivelli SM, Giovagnoni C, Visseren L, Scheithauer AL, de Wit N, den Hoedt S, et al. Sphingolipids in Alzheimer’s disease, how can we target them? Adv Drug Deliv Rev. 2020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

