Clinical Outcomes of Patients With Chronic Myeloid Leukemia With Concurrent Core Binding Factor Rearrangement and Philadelphia Chromosome
- PMID: 33597098
- PMCID: PMC11513896
- DOI: 10.1016/j.clml.2020.12.025
Clinical Outcomes of Patients With Chronic Myeloid Leukemia With Concurrent Core Binding Factor Rearrangement and Philadelphia Chromosome
Abstract
Background: Acquisition of additional cytogenetic abnormalities (ACAs) in addition to Philadelphia chromosome is frequently observed in patients with chronic myeloid leukemia (CML) in advanced phase. The presence of core binding factor (CBF) translocations determines the diagnosis of acute myeloid leukemia regardless of blast percentage, and CBF rearrangements are rarely identified as ACAs.
Patients and methods: A retrospective chart review of patients with CML who had CBF rearrangement, t(8;21) or inv(16), in Philadelphia chromosome-positive clones was conducted. Additional cases of CML with CBF rearrangements were identified through literature review.
Results: Between August 1997 and December 2014, we identified 11 patients who had Philadelphia chromosome and CBF rearrangement in the same clones: 1 (9%) with t(8;21) and 10 (91%) with inv(16). Nine (82%) patients were in blast phase, and 2 (18%) in second chronic phase. Four (36%) patients received tyrosine kinase inhibitor monotherapy, 2 (18%) received tyrosine kinase inhibitor and chemotherapy, and 5 (45%) received chemotherapy only. Three (27%) patients achieved complete remission with incomplete count recovery, and 4 (36%) had no response after the initial therapy. Three (27%) patients underwent allogeneic stem cell transplantation. The median event-free survival and overall survival for the 11 patients were 2 months and 6 months, respectively. Literature review identified 14 patients with CML with CBF rearrangement with a median overall survival of 14 months.
Conclusion: Acquisition of CBF rearrangement in addition to Philadelphia chromosome is a rare phenomenon associated with poor prognosis. CBF rearrangements as ACAs in patients with CML can be considered high-risk features.
Keywords: Additional cytogenetic abnormalities; Blast phase; Chronic myeloid leukemia; Core binding factor; Tyrosine kinase inhibitor.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Kiyomi Morita: This author declares no conflict of interest.
Elias Jabbour: This author declares research support and an advisory role with Adaptive, AbbVie, Amgen, Pfizer, Cyclacel LTD, Takeda, BMS.
Farhad Ravandi: This author declares research support and an advisory role with Macrogeni, Selvita, Cyclacel LTD, Menarini Ricerche, Xencor.
Gautam Borthakur: This author declares no conflict of interest.
Joseph D. Khoury: This author declares no conflict of interest.
Shimin Hu: This author declares no conflict of interest.
Guillermo Garcia-Manero: This author declares research support and an advisory role with Bristol Myers Squibb, Astex, and Helsinn, and research support from Amphivena, Novartis, AbbVie, H3 Biomedicine, Onconova, and Merck.
William Wierda: This author declares no conflict of interest.
Ghayas Issa: This author declares research support and an advisory role with Celegene, Syndax, and Novartis.
Naval Daver: This author declares no conflict of interest.
Naveen Pemmaraju: This author declares no conflict of interest.
Guillermo Montalban-Bravo: This author declares no conflict of interest.
Kelly A. Soltysiak: This author declares no conflict of interest.
Sherry Pierce: This author declares no conflict of interest.
Carlos Bueso-Ramos: This author declares no conflict of interest.
Jorge Cortes: This author declares research support, consultancy, and an advisory role with
Novartis, Pfizer, Takeda, Sun Pharma, BioPath Holdings, Telios, Astellas, Amphivena
Therapeutics, Arogs, BiolineRx, BMS, Daiichi Sankyo, Jazz, Immunogen, Merus, Tolero Pharmaceuticals, and Tovagene.
Koji Sasaki: This author declares an advisory role with Pfizer Japan.
Figures
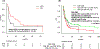
Similar articles
-
Chromosomal rearrangement involving 11q23 locus in chronic myelogenous leukemia: a rare phenomenon frequently associated with disease progression and poor prognosis.J Hematol Oncol. 2015 Apr 8;8:32. doi: 10.1186/s13045-015-0128-2. J Hematol Oncol. 2015. PMID: 25888368 Free PMC article.
-
The impact of additional cytogenetic abnormalities at diagnosis and during therapy with tyrosine kinase inhibitors in Chronic Myeloid Leukaemia.J Med Life. 2015 Oct-Dec;8(4):502-8. J Med Life. 2015. PMID: 26664479 Free PMC article.
-
Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia.Clin Ther. 2007 Nov;29(11):2289-308. doi: 10.1016/j.clinthera.2007.11.005. Clin Ther. 2007. PMID: 18158072 Review.
-
Venetoclax and BCR-ABL Tyrosine Kinase Inhibitor Combinations: Outcome in Patients with Philadelphia Chromosome-Positive Advanced Myeloid Leukemias.Acta Haematol. 2020;143(6):567-573. doi: 10.1159/000506346. Epub 2020 Apr 14. Acta Haematol. 2020. PMID: 32289808 Free PMC article.
-
Chronic myeloid leukemia with complex karyotypes: Prognosis and therapeutic approaches.J Cell Physiol. 2019 May;234(5):5798-5806. doi: 10.1002/jcp.27505. Epub 2018 Nov 14. J Cell Physiol. 2019. PMID: 30430567 Review.
Cited by
-
Adverse events and dose modifications of tyrosine kinase inhibitors in chronic myelogenous leukemia.Front Oncol. 2022 Oct 6;12:1021662. doi: 10.3389/fonc.2022.1021662. eCollection 2022. Front Oncol. 2022. PMID: 36276124 Free PMC article. Review.
-
A Review on the Therapeutic Role of TKIs in Case of CML in Combination With Epigenetic Drugs.Front Genet. 2021 Oct 22;12:742802. doi: 10.3389/fgene.2021.742802. eCollection 2021. Front Genet. 2021. PMID: 34745216 Free PMC article. Review.
-
Blast Transformation of Chronic Myeloid Leukemia Driven by Acquisition of t(8;21)(q22;q22)/RUNX1::RUNX1T1: Selecting Optimal Treatment Based on Clinical and Molecular Findings.Biomedicines. 2024 Oct 15;12(10):2339. doi: 10.3390/biomedicines12102339. Biomedicines. 2024. PMID: 39457651 Free PMC article.
-
Chromosomal Instability in Chronic Myeloid Leukemia: Mechanistic Insights and Effects.Cancers (Basel). 2022 May 21;14(10):2533. doi: 10.3390/cancers14102533. Cancers (Basel). 2022. PMID: 35626137 Free PMC article. Review.
-
Amino Acid Metabolic Vulnerabilities in Acute and Chronic Myeloid Leukemias.Front Oncol. 2021 Jul 1;11:694526. doi: 10.3389/fonc.2021.694526. eCollection 2021. Front Oncol. 2021. PMID: 34277440 Free PMC article. Review.
References
-
- Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34:2333–2340. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

