Efficacy and Safety of Bimagrumab in Sporadic Inclusion Body Myositis: Long-term Extension of RESILIENT
- PMID: 33597289
- PMCID: PMC8032371
- DOI: 10.1212/WNL.0000000000011626
Efficacy and Safety of Bimagrumab in Sporadic Inclusion Body Myositis: Long-term Extension of RESILIENT
Abstract
Objective: To assess long-term (2 years) effects of bimagrumab in participants with sporadic inclusion body myositis (sIBM).
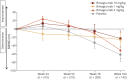
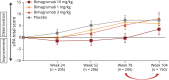
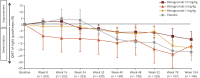
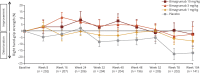
Methods: Participants (aged 36-85 years) who completed the core study (RESILIENT [Efficacy and Safety of Bimagrumab/BYM338 at 52 Weeks on Physical Function, Muscle Strength, Mobility in sIBM Patients]) were invited to join an extension study. Individuals continued on the same treatment as in the core study (10 mg/kg, 3 mg/kg, 1 mg/kg bimagrumab or matching placebo administered as IV infusions every 4 weeks). The co-primary outcome measures were 6-minute walk distance (6MWD) and safety.
Results: Between November 2015 and February 2017, 211 participants entered double-blind placebo-controlled period of the extension study. Mean change in 6MWD from baseline was highly variable across treatment groups, but indicated progressive deterioration from weeks 24-104 in all treatment groups. Overall, 91.0% (n = 142) of participants in the pooled bimagrumab group and 89.1% (n = 49) in the placebo group had ≥1 treatment-emergent adverse event (AE). Falls were slightly higher in the bimagrumab 3 mg/kg group vs 10 mg/kg, 1 mg/kg, and placebo groups (69.2% [n = 36 of 52] vs 56.6% [n = 30 of 53], 58.8% [n = 30 of 51], and 61.8% [n = 34 of 55], respectively). The most frequently reported AEs in the pooled bimagrumab group were diarrhea 14.7% (n = 23), involuntary muscle contractions 9.6% (n = 15), and rash 5.1% (n = 8). Incidence of serious AEs was comparable between the pooled bimagrumab and the placebo group (18.6% [n = 29] vs 14.5% [n = 8], respectively).
Conclusion: Extended treatment with bimagrumab up to 2 years produced a good safety profile and was well-tolerated, but did not provide clinical benefits in terms of improvement in mobility. The extension study was terminated early due to core study not meeting its primary endpoint.
Clinical trial registration: Clinicaltrials.gov identifier NCT02573467.
Classification of evidence: This study provides Class IV evidence that for patients with sIBM, long-term treatment with bimagrumab was safe, well-tolerated, and did not provide meaningful functional benefit. The study is rated Class IV because of the open-label design of extension treatment period 2.
© 2021 American Academy of Neurology.
Figures

Comment in
-
Challenges for Treatment Trials of Inclusion Body Myositis.Neurology. 2021 Mar 23;96(12):555-556. doi: 10.1212/WNL.0000000000011628. Epub 2021 Feb 17. Neurology. 2021. PMID: 33597288 No abstract available.
References
-
- Benveniste O, Guiguet M, Freebody J, et al. . Long-term observational study of sporadic inclusion body myositis. Brain 2011;134:3176–3184. - PubMed
-
- Cox FM, Titulaer MJ, Sont JK, Wintzen AR, Verschuuren JJ, Badrising UA. A 12-year follow-up in sporadic inclusion body myositis: an end stage with major disabilities. Brain 2011;134:3167–3175. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
