Automated detection of cerebral microbleeds on T2*-weighted MRI
- PMID: 33597663
- PMCID: PMC7889861
- DOI: 10.1038/s41598-021-83607-0
Automated detection of cerebral microbleeds on T2*-weighted MRI
Abstract
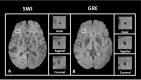
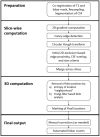
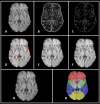
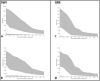
Cerebral microbleeds, observed as small, spherical hypointense regions on gradient echo (GRE) or susceptibility weighted (SWI) magnetic resonance imaging (MRI) sequences, reflect small hemorrhagic infarcts, and are associated with conditions such as vascular dementia, small vessel disease, cerebral amyloid angiopathy, and Alzheimer's disease. The current gold standard for detecting and rating cerebral microbleeds in a research context is visual inspection by trained raters, a process that is both time consuming and subject to poor reliability. We present here a novel method to automate microbleed detection on GRE and SWI images. We demonstrate in a community-based cohort of older adults that the method is highly sensitive (greater than 92% of all microbleeds accurately detected) across both modalities, with reasonable precision (fewer than 20 and 10 false positives per scan on GRE and SWI, respectively). We also demonstrate that the algorithm can be used to identify microbleeds over longitudinal scans with a higher level of sensitivity than visual ratings (50% of longitudinal microbleeds correctly labeled by the algorithm, while manual ratings was 30% or lower). Further, the algorithm identifies the anatomical localization of microbleeds based on brain atlases, and greatly reduces time spent completing visual ratings (43% reduction in visual rating time). Our automatic microbleed detection instrument is ideal for implementation in large-scale studies that include cross-sectional and longitudinal scanning, as well as being capable of performing well across multiple commonly used MRI modalities.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

