Structure and inhibition mechanism of the human citrate transporter NaCT
- PMID: 33597751
- PMCID: PMC7933130
- DOI: 10.1038/s41586-021-03230-x
Structure and inhibition mechanism of the human citrate transporter NaCT
Abstract
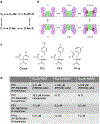
Citrate is best known as an intermediate in the tricarboxylic acid cycle of the cell. In addition to this essential role in energy metabolism, the tricarboxylate anion also acts as both a precursor and a regulator of fatty acid synthesis1-3. Thus, the rate of fatty acid synthesis correlates directly with the cytosolic concentration of citrate4,5. Liver cells import citrate through the sodium-dependent citrate transporter NaCT (encoded by SLC13A5) and, as a consequence, this protein is a potential target for anti-obesity drugs. Here, to understand the structural basis of its inhibition mechanism, we determined cryo-electron microscopy structures of human NaCT in complexes with citrate or a small-molecule inhibitor. These structures reveal how the inhibitor-which binds to the same site as citrate-arrests the transport cycle of NaCT. The NaCT-inhibitor structure also explains why the compound selectively inhibits NaCT over two homologous human dicarboxylate transporters, and suggests ways to further improve the affinity and selectivity. Finally, the NaCT structures provide a framework for understanding how various mutations abolish the transport activity of NaCT in the brain and thereby cause epilepsy associated with mutations in SLC13A5 in newborns (which is known as SLC13A5-epilepsy)6-8.
Conflict of interest statement
Competing interests
The authors declare no competing interest
Figures








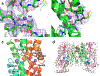


Similar articles
-
Structure and mechanism of a bacterial sodium-dependent dicarboxylate transporter.Nature. 2012 Nov 22;491(7425):622-6. doi: 10.1038/nature11542. Epub 2012 Oct 21. Nature. 2012. PMID: 23086149 Free PMC article.
-
State-Dependent Allosteric Inhibition of the Human SLC13A5 Citrate Transporter by Hydroxysuccinic Acids, PF-06649298 and PF-06761281.Mol Pharmacol. 2016 Dec;90(6):766-774. doi: 10.1124/mol.116.106575. Epub 2016 Oct 17. Mol Pharmacol. 2016. PMID: 27754898
-
Discovery and characterization of novel inhibitors of the sodium-coupled citrate transporter (NaCT or SLC13A5).Sci Rep. 2015 Dec 1;5:17391. doi: 10.1038/srep17391. Sci Rep. 2015. PMID: 26620127 Free PMC article.
-
Consequences of NaCT/SLC13A5/mINDY deficiency: good versus evil, separated only by the blood-brain barrier.Biochem J. 2021 Feb 12;478(3):463-486. doi: 10.1042/BCJ20200877. Biochem J. 2021. PMID: 33544126 Free PMC article. Review.
-
Role of sodium dependent SLC13 transporter inhibitors in various metabolic disorders.Mol Cell Biochem. 2023 Aug;478(8):1669-1687. doi: 10.1007/s11010-022-04618-7. Epub 2022 Dec 10. Mol Cell Biochem. 2023. PMID: 36495372 Review.
Cited by
-
Structural basis for inhibition of the drug efflux pump NorA from Staphylococcus aureus.Nat Chem Biol. 2022 Jul;18(7):706-712. doi: 10.1038/s41589-022-00994-9. Epub 2022 Mar 31. Nat Chem Biol. 2022. PMID: 35361990 Free PMC article.
-
Applications of Cryo-EM in small molecule and biologics drug design.Biochem Soc Trans. 2021 Dec 17;49(6):2627-2638. doi: 10.1042/BST20210444. Biochem Soc Trans. 2021. PMID: 34812853 Free PMC article.
-
NaCT/SLC13A5 facilitates citrate import and metabolism under nutrient-limited conditions.Cell Rep. 2021 Sep 14;36(11):109701. doi: 10.1016/j.celrep.2021.109701. Cell Rep. 2021. PMID: 34525352 Free PMC article.
-
Targeting SLC transporters: small molecules as modulators and therapeutic opportunities.Trends Biochem Sci. 2023 Sep;48(9):801-814. doi: 10.1016/j.tibs.2023.05.011. Epub 2023 Jun 22. Trends Biochem Sci. 2023. PMID: 37355450 Free PMC article. Review.
-
The ups and downs of elevator-type di-/tricarboxylate membrane transporters.FEBS J. 2022 Mar;289(6):1515-1523. doi: 10.1111/febs.16158. Epub 2021 Aug 24. FEBS J. 2022. PMID: 34403567 Free PMC article.
References
-
- Spencer AF & Lowenstein JM, Supply of precursors for synthesis of fatty acids. J Biol Chem 237, 3640–3648 (1962). - PubMed
-
- Ruderman NB, Saha AK, Vavvas D, & Witters LA, Malonyl-CoA, fuel sensing, and insulin resistance. Am J Physiol-Endoc M 276, E1–E18 (1999). - PubMed
-
- Sul HS & Smith S, Fatty acid synthesis in eukaryotes in Biochemistry of Lipids, Lipoproteins and Membranes, edited by Vance DE & Vance JE (Elsevier, New York, 2008), pp. 155–190.
-
- Shrago E, Spennetta T, & Gordon E, Fatty acid synthesis in human adipose tissue. J Biol Chem 244, 2761–2766 (1969). - PubMed
Additional references
-
- Slotboom DJ, Duurkens RH, Olieman K, & Erkens GB, Static light scattering to characterize membrane proteins in detergent solution. Methods 46, 73–82 (2008). - PubMed
-
- Kendrick BS, Kerwin BA, Chang BS, & Philo JS, Online size-exclusion high-performance liquid chromatography light scattering and differential refractometry methods to determine degree of polymer conjugation to proteins and protein-protein or protein-ligand association states. Anal Biochem 299, 136–146 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

