Glutamate transporters have a chloride channel with two hydrophobic gates
- PMID: 33597752
- PMCID: PMC7954978
- DOI: 10.1038/s41586-021-03240-9
Glutamate transporters have a chloride channel with two hydrophobic gates
Abstract
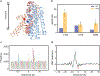
Glutamate is the most abundant excitatory neurotransmitter in the central nervous system, and its precise control is vital to maintain normal brain function and to prevent excitotoxicity1. The removal of extracellular glutamate is achieved by plasma-membrane-bound transporters, which couple glutamate transport to sodium, potassium and pH gradients using an elevator mechanism2-5. Glutamate transporters also conduct chloride ions by means of a channel-like process that is thermodynamically uncoupled from transport6-8. However, the molecular mechanisms that enable these dual-function transporters to carry out two seemingly contradictory roles are unknown. Here we report the cryo-electron microscopy structure of a glutamate transporter homologue in an open-channel state, which reveals an aqueous cavity that is formed during the glutamate transport cycle. The functional properties of this cavity, combined with molecular dynamics simulations, reveal it to be an aqueous-accessible chloride permeation pathway that is gated by two hydrophobic regions and is conserved across mammalian and archaeal glutamate transporters. Our findings provide insight into the mechanism by which glutamate transporters support their dual function, and add information that will assist in mapping the complete transport cycle shared by the solute carrier 1A transporter family.
Conflict of interest statement
Competing interest declaration
The authors declare no competing interests.
Figures



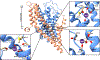





References
-
- Vandenberg RJ, Ryan RM, Mechanisms of glutamate transport. Physiol Rev 93, 1621–1657 (2013). - PubMed
-
- Ryan RM, Vandenberg RJ, Elevating the alternating-access model. Nat Struct Mol Biol 23, 187–189 (2016). - PubMed
-
- Zerangue N, Kavanaugh MP, Flux coupling in a neuronal glutamate transporter. Nature 383, 634–637 (1996). - PubMed
-
- Wadiche JI, Arriza JL, Amara SG, Kavanaugh MP, Kinetics of a human glutamate transporter. Neuron 14, 1019–1027 (1995). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

