Loop extrusion as a mechanism for formation of DNA damage repair foci
- PMID: 33597753
- PMCID: PMC7116834
- DOI: 10.1038/s41586-021-03193-z
Loop extrusion as a mechanism for formation of DNA damage repair foci
Abstract
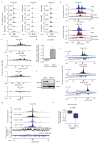
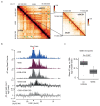
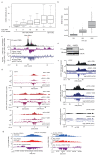
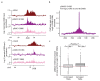
The repair of DNA double-strand breaks (DSBs) is essential for safeguarding genome integrity. When a DSB forms, the PI3K-related ATM kinase rapidly triggers the establishment of megabase-sized, chromatin domains decorated with phosphorylated histone H2AX (γH2AX), which act as seeds for the formation of DNA-damage response foci1. It is unclear how these foci are rapidly assembled to establish a 'repair-prone' environment within the nucleus. Topologically associating domains are a key feature of 3D genome organization that compartmentalize transcription and replication, but little is known about their contribution to DNA repair processes2,3. Here we show that topologically associating domains are functional units of the DNA damage response, and are instrumental for the correct establishment of γH2AX-53BP1 chromatin domains in a manner that involves one-sided cohesin-mediated loop extrusion on both sides of the DSB. We propose a model in which H2AX-containing nucleosomes are rapidly phosphorylated as they actively pass by DSB-anchored cohesin. Our work highlights the importance of chromosome conformation in the maintenance of genome integrity and demonstrates the establishment of a chromatin modification by loop extrusion.
Conflict of interest statement
The authors declare no competing interests.
Figures





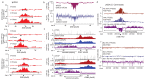
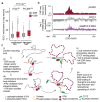
Comment in
-
Cells use loop extrusion to weave and tie the genome.Nature. 2021 Feb;590(7847):554-555. doi: 10.1038/d41586-021-00351-1. Nature. 2021. PMID: 33597775 No abstract available.
References
-
- Clouaire T, Marnef A, Legube G. Taming Tricky DSBs: ATM on duty. DNA Repair (Amst) 2017;56:84–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

