Efficacy and Safety of Daprodustat for Anemia Therapy in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis
- PMID: 33597868
- PMCID: PMC7883598
- DOI: 10.3389/fphar.2020.573645
Efficacy and Safety of Daprodustat for Anemia Therapy in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis
Abstract
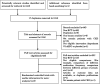
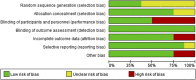
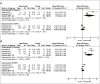
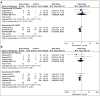
Objective: Daprodustat is a novel oral agent in treating anemia of chronic kidney disease (CKD), and several clinical trials have been conducted to compare daprodustat with recombinant human erythropoietin (rhEPO) or placebo. Our systematic review aimed to investigate the efficacy and safety of daprodustat for anemia treatment in both dialysis-dependent (DD) and non-dialysis-dependent (NDD) patients. Methods: Six databases were searched for randomized controlled trials (RCTs) reporting daprodustat vs. rhEPO or placebo for anemia patients in CKD. The outcome indicators were focused on hemoglobin (Hb), ferritin, transferrin saturation (TSAT), total iron-binding capacity (TIBC), vascular endothelial growth factor (VEGF), and serious adverse events (SAEs). Results: Eight eligible studies with 1,516 participants were included. For both NDD and DD patients, changes in Hb levels from baseline were significantly higher in daprodustat group than that in the placebo (mean difference (MD) = 1.73, [95% confidence interval (CI), 0.34 to 3.12], p = 0.01; MD = 1.88, [95% CI, 0.68 to 3.09], p = 0.002; respectively), and there was no significant difference between daprodustat and rhEPO group (MD = 0.05, [95% CI, -0.49 to 0.59], p = 0.86; MD = 0.12, [95% CI, -0.28 to 0.52], p = 0.55; respectively). The indexes of iron metabolism were improved significantly in the daprodustat group compared to placebo- or rhEPO-treated patients, while there was no similar change in terms of TSAT for DD patients. Furthermore, no trend of increasing plasma VEGF was observed in daprodustat-treated subjects. As for safety, there was no significant difference in the incidence of SAEs between daprodustat and placebo treatment, while the incidence of SAEs in the daprodustat group was significantly lower than that in the rhEPO group. Conclusion: Daprodustat was efficacious and well tolerated for anemia in both NDD and DD patients in the short term based on current RCTs. And daprodustat may become an effective alternative for treatment of anemia with CKD. Since the application of daprodustat is still under exploration, future researches should consider the limitations of our study to evaluate the value of daprodustat.
Keywords: anemia; chronic kidney disease; daprodustat; meta-analysis; systematic review.
Copyright © 2021 Zheng, Wang, Yang, Sun, Fu, Wei, Liu and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Efficacy and Safety of Daprodustat Vs rhEPO for Anemia in Patients With Chronic Kidney Disease: A Meta-Analysis and Trial Sequential Analysis.Front Pharmacol. 2022 Mar 10;13:746265. doi: 10.3389/fphar.2022.746265. eCollection 2022. Front Pharmacol. 2022. PMID: 35359863 Free PMC article.
-
Evaluating the safety and efficacy of daprodustat for anemia of chronic kidney disease: a meta-analysis of randomized clinical trials.Eur J Clin Pharmacol. 2022 Dec;78(12):1867-1875. doi: 10.1007/s00228-022-03395-y. Epub 2022 Oct 5. Eur J Clin Pharmacol. 2022. PMID: 36195739 Review.
-
Efficacy and safety of vadadustat compared to darbepoetin alfa on anemia in patients with chronic kidney disease: a meta-analysis.Int Urol Nephrol. 2023 Feb;55(2):325-334. doi: 10.1007/s11255-022-03316-z. Epub 2022 Aug 12. Int Urol Nephrol. 2023. PMID: 35960479 Review.
-
The efficacy and safety of roxadustat for anemia in patients with chronic kidney disease: a meta-analysis.Nephrol Dial Transplant. 2021 Aug 27;36(9):1603-1615. doi: 10.1093/ndt/gfaa110. Nephrol Dial Transplant. 2021. PMID: 33051677
-
The Efficacy and Safety of Roxadustat for Anemia in Patients With Chronic Kidney Disease: A Meta-Analysis.Front Pharmacol. 2022 Apr 26;13:779694. doi: 10.3389/fphar.2022.779694. eCollection 2022. Front Pharmacol. 2022. PMID: 35559232 Free PMC article.
Cited by
-
Efficacy and Safety of Daprodustat Vs rhEPO for Anemia in Patients With Chronic Kidney Disease: A Meta-Analysis and Trial Sequential Analysis.Front Pharmacol. 2022 Mar 10;13:746265. doi: 10.3389/fphar.2022.746265. eCollection 2022. Front Pharmacol. 2022. PMID: 35359863 Free PMC article.
-
Efficacy of Different Doses of Daprodustat for Anemic Non-dialysis Patients with Chronic Kidney Disease: A Systematic Review and Network Meta-Analysis.J Clin Med. 2022 May 11;11(10):2722. doi: 10.3390/jcm11102722. J Clin Med. 2022. PMID: 35628849 Free PMC article. Review.
-
Role of serum β2-microglobulin, glycosylated hemoglobin, and vascular endothelial growth factor levels in diabetic nephropathy.World J Clin Cases. 2022 Aug 16;10(23):8205-8211. doi: 10.12998/wjcc.v10.i23.8205. World J Clin Cases. 2022. PMID: 36159531 Free PMC article.
-
Comparative effectiveness and acceptability of HIF prolyl-hydroxylase inhibitors versus for anemia patients with chronic kidney disease undergoing dialysis: a systematic review and network meta-analysis.Front Pharmacol. 2023 Jul 13;14:1050412. doi: 10.3389/fphar.2023.1050412. eCollection 2023. Front Pharmacol. 2023. PMID: 37521459 Free PMC article.
-
Evaluating the safety and efficacy of daprodustat for anemia of chronic kidney disease: a meta-analysis of randomized clinical trials.Eur J Clin Pharmacol. 2022 Dec;78(12):1867-1875. doi: 10.1007/s00228-022-03395-y. Epub 2022 Oct 5. Eur J Clin Pharmacol. 2022. PMID: 36195739 Review.
References
-
- Ariazi J. L., Duffy K. J., Adams D. F., Fitch D. M., Luo L., Pappalardi M., et al. (2017). Discovery and preclinical characterization of GSK1278863 (daprodustat), a small molecule hypoxia inducible factor-prolyl hydroxylase inhibitor for anemia. J. Pharmacol. Exp. Ther. 363, 336–347. 10.1124/jpet.117.242503 - DOI - PubMed
-
- Bailey C. K., Caltabiano S., Cobitz A. R., Huang C., Mahar K. M., Patel V. V. (2019). A randomized, 29-day, dose-ranging, efficacy and safety study of daprodustat, administered three times weekly in patients with anemia on hemodialysis. BMC Nephrol. 20, 372 10.1186/s12882-019-1547-z - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

