The Role of Lipophagy in the Development and Treatment of Non-Alcoholic Fatty Liver Disease
- PMID: 33597924
- PMCID: PMC7883485
- DOI: 10.3389/fendo.2020.601627
The Role of Lipophagy in the Development and Treatment of Non-Alcoholic Fatty Liver Disease
Abstract
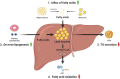
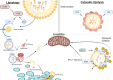
Non-alcoholic fatty liver disease (NAFLD) or metabolic (dysfunction) associated liver disease (MAFLD), is, with a global prevalence of 25%, the most common liver disorder worldwide. NAFLD comprises a spectrum of liver disorders ranging from simple steatosis to steatohepatitis, fibrosis, cirrhosis and eventually end-stage liver disease. The cause of NAFLD is multifactorial with genetic susceptibility and an unhealthy lifestyle playing a crucial role in its development. Disrupted hepatic lipid homeostasis resulting in hepatic triglyceride accumulation is an hallmark of NAFLD. This disruption is commonly described based on four pathways concerning 1) increased fatty acid influx, 2) increased de novo lipogenesis, 3) reduced triglyceride secretion, and 4) reduced fatty acid oxidation. More recently, lipophagy has also emerged as pathway affecting NAFLD development and progression. Lipophagy is a form of autophagy (i.e. controlled autolysosomal degradation and recycling of cellular components), that controls the breakdown of lipid droplets in the liver. Here we address the role of hepatic lipid homeostasis in NAFLD and specifically review the current literature on lipophagy, describing its underlying mechanism, its role in pathophysiology and its potential as a therapeutic target.
Keywords: autophagy; lipid droplet; lipid homeostasis; lipophagy; non-alcoholic fatty liver disease.
Copyright © 2021 Grefhorst, van de Peppel, Larsen, Jonker and Holleboom.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

