Simvastatin and ROCK Inhibitor Y-27632 Inhibit Myofibroblast Differentiation of Graves' Ophthalmopathy-Derived Orbital Fibroblasts via RhoA-Mediated ERK and p38 Signaling Pathways
- PMID: 33597925
- PMCID: PMC7883643
- DOI: 10.3389/fendo.2020.607968
Simvastatin and ROCK Inhibitor Y-27632 Inhibit Myofibroblast Differentiation of Graves' Ophthalmopathy-Derived Orbital Fibroblasts via RhoA-Mediated ERK and p38 Signaling Pathways
Abstract
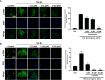
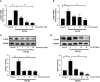
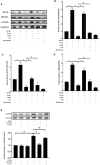
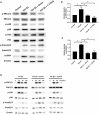
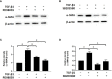
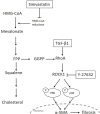
Transforming growth factor-β (TGF-β)-induced differentiation of orbital fibroblasts into myofibroblasts is an important pathogenesis of Graves' ophthalmopathy (GO) and leads to orbital tissue fibrosis. In the present study, we explored the antifibrotic effects of simvastatin and ROCK inhibitor Y-27632 in primary cultured GO orbital fibroblasts and tried to explain the molecular mechanisms behind these effects. Both simvastatin and Y-27632 inhibited TGF-β-induced α-smooth muscle actin (α-SMA) expression, which serves as a marker of fibrosis. The inhibitory effect of simvastatin on TGF-β-induced RhoA, ROCK1, and α-SMA expression could be reversed by geranylgeranyl pyrophosphate, an intermediate in the biosynthesis of cholesterol. This suggested that the mechanism of simvastatin-mediated antifibrotic effects may involve RhoA/ROCK signaling. Furthermore, simvastatin and Y-27632 suppressed TGF-β-induced phosphorylation of ERK and p38. The TGF-β-mediated α-SMA expression was suppressed by pharmacological inhibitors of p38 and ERK. These results suggested that simvastatin inhibits TGF-β-induced myofibroblast differentiation via suppression of the RhoA/ROCK/ERK and p38 MAPK signaling pathways. Thus, our study provides evidence that simvastatin and ROCK inhibitors may be potential therapeutic drugs for the prevention and treatment of orbital fibrosis in GO.
Keywords: ERK; Graves’ ophthalmopathy; Ras homolog family member A (RhoA); Rho‑associated protein kinase (ROCK); Y-27632; myofibroblast; p38; simvastatin.
Copyright © 2021 Wei, Liao, Wang, Wang and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Effect of simvastatin on transforming growth factor beta-1-induced myofibroblast differentiation and collagen production in nasal polyp-derived fibroblasts.Am J Rhinol Allergy. 2012 Jan-Feb;26(1):7-11. doi: 10.2500/ajra.2012.26.3679. Am J Rhinol Allergy. 2012. PMID: 22391067
-
JNK and p38 Inhibitors Prevent Transforming Growth Factor-β1-Induced Myofibroblast Transdifferentiation in Human Graves' Orbital Fibroblasts.Int J Mol Sci. 2021 Mar 14;22(6):2952. doi: 10.3390/ijms22062952. Int J Mol Sci. 2021. PMID: 33799469 Free PMC article.
-
Effect of Pirfenidone on TGF-β1-Induced Myofibroblast Differentiation and Extracellular Matrix Homeostasis of Human Orbital Fibroblasts in Graves' Ophthalmopathy.Biomolecules. 2021 Sep 29;11(10):1424. doi: 10.3390/biom11101424. Biomolecules. 2021. PMID: 34680057 Free PMC article.
-
A Review of Pathophysiology and Therapeutic Strategies Targeting TGF-β in Graves' Ophthalmopathy.Cells. 2024 Sep 5;13(17):1493. doi: 10.3390/cells13171493. Cells. 2024. PMID: 39273063 Free PMC article. Review.
-
Molecular biomarkers of Graves' ophthalmopathy.Exp Mol Pathol. 2019 Feb;106:1-6. doi: 10.1016/j.yexmp.2018.11.004. Epub 2018 Nov 8. Exp Mol Pathol. 2019. PMID: 30414981 Free PMC article. Review.
Cited by
-
RhoA with Associated TRAb or FT3 in the Diagnosis and Prediction of Graves' Ophthalmopathy.Dis Markers. 2022 Jul 29;2022:8323946. doi: 10.1155/2022/8323946. eCollection 2022. Dis Markers. 2022. PMID: 35937945 Free PMC article.
-
The essential role of N6-methyladenosine RNA methylation in complex eye diseases.Genes Dis. 2022 May 26;10(2):505-520. doi: 10.1016/j.gendis.2022.05.008. eCollection 2023 Mar. Genes Dis. 2022. PMID: 37223523 Free PMC article. Review.
-
IL-11 Is Elevated and Drives the Profibrotic Phenotype Transition of Orbital Fibroblasts in Thyroid-Associated Ophthalmopathy.Front Endocrinol (Lausanne). 2022 Feb 22;13:846106. doi: 10.3389/fendo.2022.846106. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35273577 Free PMC article.
-
Traditional Chinese medicine in thyroid-associated orbitopathy.J Endocrinol Invest. 2023 Jun;46(6):1103-1113. doi: 10.1007/s40618-023-02024-4. Epub 2023 Feb 12. J Endocrinol Invest. 2023. PMID: 36781592 Free PMC article. Review.
-
Cytoplasmic Clusterin Suppresses Lung Cancer Metastasis by Inhibiting the ROCK1-ERK Axis.Cancers (Basel). 2022 May 17;14(10):2463. doi: 10.3390/cancers14102463. Cancers (Basel). 2022. PMID: 35626071 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

