Pathogenesis and Immune Response of Ebinur Lake Virus: A Newly Identified Orthobunyavirus That Exhibited Strong Virulence in Mice
- PMID: 33597934
- PMCID: PMC7882632
- DOI: 10.3389/fmicb.2020.625661
Pathogenesis and Immune Response of Ebinur Lake Virus: A Newly Identified Orthobunyavirus That Exhibited Strong Virulence in Mice
Abstract
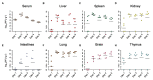
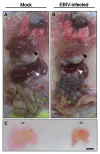
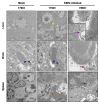
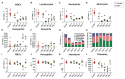
Orthobunyaviruses are a group of viruses with significant public and veterinary health importance. These viruses are mainly transmitted through mosquito-, midge-, and tick-vectors, and are endemic to various regions of the world. Ebinur Lake virus (EBIV), a newly identified member of Orthobunyavirus, was isolated from Culex mosquitoes in Northwest China. In the present study, we aimed to characterize the pathogenesis and host immune responses of EBIV in BALB/c mice, as an animal model. Herein, we determined that BALB/c mice are highly susceptible to EBIV infection. The infected mice exhibited evident clinical signs including weight loss, mild encephalitis, and death. High mortality of mice was observed even with inoculation of one plaque-forming unit (PFU) of EBIV, and the infected mice succumbed to death within 5-9 days. After EBIV challenge, rapid viremic dissemination was detected in the peripheral tissues and the central nervous system, with prominent histopathologic changes observed in liver, spleen, thymus, and brain. Blood constituents' analysis of EBIV infected mice exhibited leukopenia, thrombocytopenia, and significantly elevated ALT, LDH-L, and CK. Further, EBIV infection induced obvious cytokines changes in serum, spleen, and brain in mice. Collectively, our data describe the first study that systematically examines the pathogenesis of EBIV and induced immune response in an immunocompetent standard mouse model, expanding our knowledge of this virus, which may pose a threat to One Health.
Keywords: BALB/c mouse; Ebinur Lake virus; Orthobunyavirus; immune response; pathogenesis.
Copyright © 2021 Zhao, Luo, Huang, Yu, Dong, Mwaliko, Atoni, Nyaruaba, Yuan, Zhang, Bente, Yuan and Xia.
Conflict of interest statement
GZ was employed by the company Xinjiang Heribase Biotechnology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Vector competence and immune response of Aedes aegypti for Ebinur Lake virus, a newly classified mosquito-borne orthobunyavirus.PLoS Negl Trop Dis. 2022 Jul 18;16(7):e0010642. doi: 10.1371/journal.pntd.0010642. eCollection 2022 Jul. PLoS Negl Trop Dis. 2022. PMID: 35849620 Free PMC article.
-
Characterization of Ebinur Lake Virus and Its Human Seroprevalence at the China-Kazakhstan Border.Front Microbiol. 2020 Jan 30;10:3111. doi: 10.3389/fmicb.2019.03111. eCollection 2019. Front Microbiol. 2020. PMID: 32082268 Free PMC article.
-
Efficient rescue of a newly classified Ebinur lake orthobunyavirus with GFP reporter and its application in rapid antiviral screening.Antiviral Res. 2022 Nov;207:105421. doi: 10.1016/j.antiviral.2022.105421. Epub 2022 Sep 21. Antiviral Res. 2022. PMID: 36150523
-
Mosquito-borne viruses in western Europe: a review.J Vector Ecol. 1999 Jun;24(1):1-39. J Vector Ecol. 1999. PMID: 10436876 Review.
-
Throw out the Map: Neuropathogenesis of the Globally Expanding California Serogroup of Orthobunyaviruses.Viruses. 2019 Aug 29;11(9):794. doi: 10.3390/v11090794. Viruses. 2019. PMID: 31470541 Free PMC article. Review.
Cited by
-
Innate Immune Response Against Batai Virus, Bunyamwera Virus, and Their Reassortants.Viruses. 2024 Nov 26;16(12):1833. doi: 10.3390/v16121833. Viruses. 2024. PMID: 39772143 Free PMC article.
-
In vivo and in vitro characterization of a new Oya virus isolate from Culicoides spp. and its seroprevalence in domestic animals in Yunnan, China.PLoS Negl Trop Dis. 2023 Jun 15;17(6):e0011374. doi: 10.1371/journal.pntd.0011374. eCollection 2023 Jun. PLoS Negl Trop Dis. 2023. PMID: 37319258 Free PMC article.
-
Insights into Two-Metal-Ion Catalytic Mechanism of Cap-Snatching Endonuclease of Ebinur Lake Virus in Bunyavirales.J Virol. 2022 Mar 23;96(6):e0208521. doi: 10.1128/jvi.02085-21. Epub 2022 Jan 19. J Virol. 2022. PMID: 35044209 Free PMC article.
-
Structural and Biochemical Basis for Development of Diketo Acid Inhibitors Targeting the Cap-Snatching Endonuclease of the Ebinur Lake Virus (Order: Bunyavirales).J Virol. 2022 Apr 13;96(7):e0217321. doi: 10.1128/jvi.02173-21. Epub 2022 Mar 10. J Virol. 2022. PMID: 35266805 Free PMC article.
-
Vector competence and immune response of Aedes aegypti for Ebinur Lake virus, a newly classified mosquito-borne orthobunyavirus.PLoS Negl Trop Dis. 2022 Jul 18;16(7):e0010642. doi: 10.1371/journal.pntd.0010642. eCollection 2022 Jul. PLoS Negl Trop Dis. 2022. PMID: 35849620 Free PMC article.
References
-
- Calisher C. H. (1996). “History, classification, and taxonomy of viruses in the family Bunyaviridae” in The Bunyaviridae. The Viruses. ed. Elliott R. M. (Boston, MA: Springer; ), 1–17.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

