Geographic Mosaics of Fly Pollinators With Divergent Color Preferences Drive Landscape-Scale Structuring of Flower Color in Daisy Communities
- PMID: 33597961
- PMCID: PMC7882612
- DOI: 10.3389/fpls.2021.617761
Geographic Mosaics of Fly Pollinators With Divergent Color Preferences Drive Landscape-Scale Structuring of Flower Color in Daisy Communities
Abstract
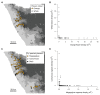
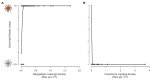
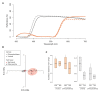
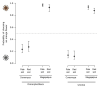
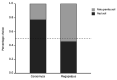
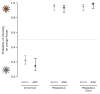
The striking variation in flower color across and within Angiosperm species is often attributed to divergent selection resulting from geographic mosaics of pollinators with different color preferences. Despite the importance of pollinator mosaics in driving floral divergence, the distributions of pollinators and their color preferences are seldom quantified. The extensive mass-flowering displays of annual daisy species in Namaqualand, South Africa, are characterized by striking color convergence within communities, but also color turnover within species and genera across large geographic scales. We aimed to determine whether shifts between orange and white-flowered daisy communities are driven by the innate color preferences of different pollinators or by soil color, which can potentially affect the detectability of different colored flowers. Different bee-fly pollinators dominated in both community types so that largely non-overlapping pollinator distributions were strongly associated with different flower colors. Visual modeling demonstrated that orange and white-flowered species are distinguishable in fly vision, and choice experiments demonstrated strongly divergent color preferences. We found that the dominant pollinator in orange communities has a strong spontaneous preference for orange flowers, which was not altered by conditioning. Similarly, the dominant pollinator in white communities exhibited an innate preference for white flowers. Although detectability of white flowers varied across soil types, background contrast did not alter color preferences. These findings demonstrate that landscape-level flower color turnover across Namaqua daisy communities is likely shaped by a strong qualitative geographic mosaic of bee-fly pollinators with divergent color preferences. This is an unexpected result given the classically generalist pollination phenotype of daisies. However, because of the dominance of single fly pollinator species within communities, and the virtual absence of bees as pollinators, we suggest that Namaqua daisies function as pollination specialists despite their generalist phenotypes, thus facilitating differentiation of flower color by pollinator shifts across the fly pollinator mosaic.
Keywords: Asteraceae (compositae); Diptera; South Africa; flower color; geographic mosaics of pollinators; greater cape floristic region; pollinator driven divergence; sensory drive.
Copyright © 2021 Ellis, Anderson and Kemp.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Born J., Linder H. P., Desmet P. (2007). The greater cape floristic region. J. Biogeogr. 34, 147–162. 10.1111/j.1365-2699.2006.01595.x - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

