CDK6 Is a Potential Prognostic Biomarker in Acute Myeloid Leukemia
- PMID: 33597968
- PMCID: PMC7882723
- DOI: 10.3389/fgene.2020.600227
CDK6 Is a Potential Prognostic Biomarker in Acute Myeloid Leukemia
Abstract
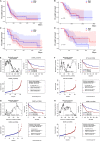
Acute myeloid leukemia (AML) is a threatening hematological malignant disease in which new successful approaches in therapy are needed. Cyclin-dependent kinase 6 (CDK6), a regulatory enzyme of the cell cycle that plays an important role in leukemogenesis and the maintenance of leukemia stem cells (LSC), has the potential to predict the prognosis of AML. By analyzing public databases, we observed that the messenger RNA (mRNA) levels of CDK6 were significantly overexpressed in AML cell lines and non-acute promyelocytic leukemia (non-APL) AML patients when compared to healthy donors. Furthermore, CDK6 expression was significantly reduced in AML patients who achieved complete remission (CR) compared to that at the time of diagnosis in our validated cohort. The expression of CDK6 was tightly correlated with peripheral blood blasts, French-American-British (FAB) subtypes, CCAAT-enhancer-binding protein α (CEBPA) mutation, and chromosomal abnormalities of t(8;21). However, the clinical significance and effects of CDK6 expression on the prognosis of non-APL AML patients remain uncertain. We found that CDK6 expression was inversely correlated with overall survival (OS) among non-APL AML patients using the Kaplan-Meier analysis. CDK6 was also found to be positively associated with genes identified to contribute to the development of leukemia, including CCND2, DNMT3B, SOX4, and IKZF2, as well as being negatively associated with anticancer microRNAs, including miR-187, miR-9, miR-582, miR708, and miR-362. In summary, our study revealed that CDK6 might be a potential diagnostic and prognostic biomarker in non-APL AML patients.
Keywords: AML; CDK6; expression; prognosis; target therapy.
Copyright © 2021 Liu, Yi, Liu, Chen, Zhang, Zhou, Zhan, Zhao, Morales, Zhao and Zeng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

