Treatment-Emergent Neuroendocrine Prostate Cancer: A Clinicopathological and Immunohistochemical Analysis of 94 Cases
- PMID: 33598420
- PMCID: PMC7882702
- DOI: 10.3389/fonc.2020.571308
Treatment-Emergent Neuroendocrine Prostate Cancer: A Clinicopathological and Immunohistochemical Analysis of 94 Cases
Abstract
Purpose: This study aimed to evaluate the pathological characteristics, immunophenotype, and prognosis of treatment-emergent neuroendocrine prostate cancer (T-NEPC).
Materials and methods: We collected 231 repeated biopsy specimens of castration-resistant prostate cancer (CRPC) cases between 2008 and 2019. We used histopathological and immunohistochemical evaluations of Synaptophysin (SYN), ChromograninA (CgA), CD56, androgen receptor (AR), and prostate-specific antigen (PSA) to screen out T-NEPC cases. Multivariate analyses were performed to identify factors in the prognosis of T-NEPC. Further, the results were verified in the Surveillance, Epidemiology, and End Results (SEER) program.
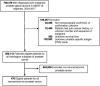
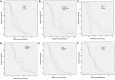
Results: Among the 231 CRPC cases, 94 (40.7%) cases were T-NEPC. T-NEPC were more likely to present with negative immunohistochemistry for AR (30.9%) and PSA (47.9%) than that of CRPC (8.8% and 17.5%, respectively). Kaplan-Meier analysis revealed that patients with T-NEPC (median overall survival [OS]: 17.6 months, 95% CI: 15.3-19.9 months) had significantly worse survival compared with usual CRPC patients (median OS: 23.6 months, 95% CI: 21.3-25.9 months, log-rank P = 0.001), especially in metastasis cases (median OS: 15.7 months, 95% CI: 13.3-18.0 months) and patients with small cell carcinoma component (median OS: 9.7 months, 95% CI: 8.2-11.2 months). Prostate adenocarcinoma with diffuse NE differentiation (median OS: 18.8 months, 95% CI: 15.3-22.3 months) had poor outcome than those with usual CRPC (P = 0.027), while there was no significant change in the focal NE differentiation (median OS: 22.9 months, 95% CI: 18.1-27.7 months, P = 0.136). In the unadjusted model, an excess risk of overall death was observed in T-NEPC with PSA negative (HR = 2.86, 95% CI = 1.39-6.73). Among 476 NEPC cases in the SEER database from 2004 to 2017, we observed a higher hazard of overall death in patients aged 65 years and older (HR = 1.35, 95% CI = 1.08-1.69), patients with PSA ≤ 2.5 ng/ml (HR = 1.90, 95%CI = 1.44-2.52), patients with PSA 2.6-4.0 ng/ml (HR = 2.03, 95%CI = 1.38-2.99), stage IV tumor (HR = 2.13, 95%CI = 1.47-3.08) and other races (HR = 1.85, 95%CI = 1.17-2.94) in total NEPC, adjusting for confounders. Similar hazard ratios were observed in pure NEPC, while there was no significant results among prostate adenocarcinoma with NE differentiation tumors.
Conclusion: T-NEPC was associated with an unfavorable prognosis, negative immunohistochemistry for PSA in T-NEPC and serum PSA level ≤ 4 ng/ml had a worse prognosis. Urologists and pathologists should recognize the importance of the second biopsy in CRPC to avoid unnecessary diagnosis and treatment delays.
Keywords: SEER program; castration-resistant prostate cancer; immunohistochemistry; small cell carcinoma; treatment-emergent neuroendocrine prostate cancer.
Copyright © 2021 Zhang, Han, Zhang, Liu, Ming, Huang and Qiu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Low-serum prostate-specific antigen level predicts poor outcomes in patients with primary neuroendocrine prostate cancer.Prostate. 2019 Sep;79(13):1563-1571. doi: 10.1002/pros.23878. Epub 2019 Aug 2. Prostate. 2019. PMID: 31376193
-
Neuroendocrine Carcinoma as an Independent Prognostic Factor for Patients With Prostate Cancer: A Population-Based Study.Front Endocrinol (Lausanne). 2021 Dec 8;12:778758. doi: 10.3389/fendo.2021.778758. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34956090 Free PMC article.
-
Real-world Clinical Outcomes and Prognostic Factors in Neuroendocrine Prostate Cancer.Clin Genitourin Cancer. 2025 Feb;23(1):102274. doi: 10.1016/j.clgc.2024.102274. Epub 2024 Nov 23. Clin Genitourin Cancer. 2025. PMID: 39689666
-
Prognostic Value of Androgen Receptor Splice Variant 7 in the Treatment of Castration-resistant Prostate Cancer with Next generation Androgen Receptor Signal Inhibition: A Systematic Review and Meta-analysis.Eur Urol Focus. 2018 Jul;4(4):529-539. doi: 10.1016/j.euf.2017.01.004. Epub 2017 Jan 23. Eur Urol Focus. 2018. PMID: 28753843
-
Molecular model for neuroendocrine prostate cancer progression.BJU Int. 2018 Oct;122(4):560-570. doi: 10.1111/bju.14207. Epub 2018 Apr 24. BJU Int. 2018. PMID: 29569310 Review.
Cited by
-
Aggressive variant prostate cancer: A case report and literature review.World J Clin Cases. 2023 Sep 16;11(26):6213-6222. doi: 10.12998/wjcc.v11.i26.6213. World J Clin Cases. 2023. PMID: 37731555 Free PMC article.
-
Effective therapeutic targeting of tumor lineage plasticity in neuroendocrine prostate cancer by BRD4 inhibitors.Acta Pharm Sin B. 2025 Mar;15(3):1415-1429. doi: 10.1016/j.apsb.2025.01.007. Epub 2025 Jan 22. Acta Pharm Sin B. 2025. PMID: 40370549 Free PMC article.
-
Advances in neuroendocrine prostate cancer research: From model construction to molecular network analyses.Lab Invest. 2022 Apr;102(4):332-340. doi: 10.1038/s41374-021-00716-0. Epub 2021 Dec 22. Lab Invest. 2022. PMID: 34937865 Review.
-
AR antagonists develop drug resistance through TOMM20 autophagic degradation-promoted transformation to neuroendocrine prostate cancer.J Exp Clin Cancer Res. 2023 Aug 10;42(1):204. doi: 10.1186/s13046-023-02776-0. J Exp Clin Cancer Res. 2023. PMID: 37563661 Free PMC article.
-
Single-cell transcriptional regulation and genetic evolution of neuroendocrine prostate cancer.iScience. 2022 Jun 13;25(7):104576. doi: 10.1016/j.isci.2022.104576. eCollection 2022 Jul 15. iScience. 2022. PMID: 35789834 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

