Role of zinc in female reproduction
- PMID: 33598687
- PMCID: PMC8599883
- DOI: 10.1093/biolre/ioab023
Role of zinc in female reproduction
Abstract
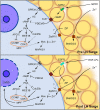
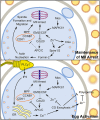
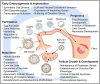
Zinc is a critical component in a number of conserved processes that regulate female germ cell growth, fertility, and pregnancy. During follicle development, a sufficient intracellular concentration of zinc in the oocyte maintains meiotic arrest at prophase I until the germ cell is ready to undergo maturation. An adequate supply of zinc is necessary for the oocyte to form a fertilization-competent egg as dietary zinc deficiency or chelation of zinc disrupts maturation and reduces the oocyte quality. Following sperm fusion to the egg to initiate the acrosomal reaction, a quick release of zinc, known as the zinc spark, induces egg activation in addition to facilitating zona pellucida hardening and reducing sperm motility to prevent polyspermy. Symmetric division, proliferation, and differentiation of the preimplantation embryo rely on zinc availability, both during the oocyte development and post-fertilization. Further, the fetal contribution to the placenta, fetal limb growth, and neural tube development are hindered in females challenged with zinc deficiency during pregnancy. In this review, we discuss the role of zinc in germ cell development, fertilization, and pregnancy with a focus on recent studies in mammalian females. We further detail the fundamental zinc-mediated reproductive processes that have only been explored in non-mammalian species and speculate on the role of zinc in similar mechanisms of female mammals. The evidence collected over the last decade highlights the necessity of zinc for normal fertility and healthy pregnancy outcomes, which suggests zinc supplementation should be considered for reproductive age women at risk of zinc deficiency.
Keywords: female; mammal; zinc.
© The Author(s) 2021. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Swenerton H, Shrader R, Hurley LS. Zinc-deficient embryos: Reduced thymidine incorporation. Science 1969; 166:1014–1015. - PubMed
-
- Duncan JR, Hurley LS. Thymidine kinase and DNA polymerase activity in normal and zinc deficient developing rat embryos. Proc Soc Exp Biol Med 1978; 159:39–43. - PubMed
-
- Wallwork JC, Duerre JA. Effect of zinc deficiency on methionine metabolism, methylation reactions and protein synthesis in isolated perfused rat liver. J Nutr 1985; 115:252–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

