In vivo emergence of beige-like fat in chickens as physiological adaptation to cold environments
- PMID: 33598768
- PMCID: PMC7979618
- DOI: 10.1007/s00726-021-02953-5
In vivo emergence of beige-like fat in chickens as physiological adaptation to cold environments
Abstract
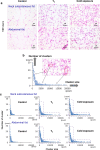
While it has been hypothesized that brown adipocytes responsible for mammalian thermogenesis are absent in birds, the existence of beige fat has yet to be studied directly. The present study tests the hypothesis that beige fat emerges in birds as a mechanism of physiological adaptation to cold environments. Subcutaneous neck adipose tissue from cold-acclimated or triiodothyronine (T3)-treated chickens exhibited increases in the expression of avian uncoupling protein (avUCP, an ortholog of mammalian UCP2 and UCP3) gene and some known mammalian beige adipocyte-specific markers. Morphological characteristics of white adipose tissues of treated chickens showed increased numbers of both small and larger clusters of multilocular fat cells within the tissues. Increases in protein levels of avUCP and mitochondrial marker protein, voltage-dependent anion channel, and immunohistochemical analysis for subcutaneous neck fat revealed the presence of potentially thermogenic mitochondria-rich cells. This is the first evidence that the capacity for thermogenesis may be acquired by differentiating adipose tissue into beige-like fat for maintaining temperature homeostasis in the subcutaneous fat 'neck warmer' in chickens exposed to a cold environment.
Keywords: Avian models; Beige fat; Cold adaptation; Thermogenesis; avUCP.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Azad KMA, Kikusato M, Hoque AM, Toyomizu M. Effect of chronic heat stress on performance and oxidative damage in different strains of chickens. J Poultry Sci. 2010;47(4):333–337. doi: 10.2141/jpsa.010025. - DOI
-
- Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metabol. 2010;298(6):E1244–1253. doi: 10.1152/ajpendo.00600.2009. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

