Macaque monkey trigeminal blink reflex circuits targeting orbicularis oculi motoneurons
- PMID: 33598920
- PMCID: PMC8164985
- DOI: 10.1002/cne.25130
Macaque monkey trigeminal blink reflex circuits targeting orbicularis oculi motoneurons
Abstract
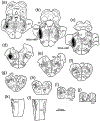
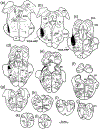
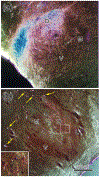
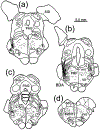
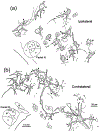
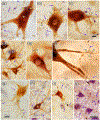
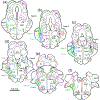
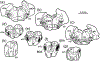
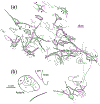
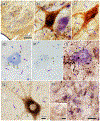
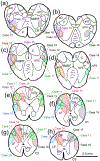
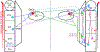
The trigeminal blink reflex plays an important role in protecting the corneal surface from damage and preserving visual function in an unpredictable environment. The closing phase of the human reflex, produced by activation of the orbicularis oculi (ObOc) muscles, consists of an initial, small, ipsilateral R1 component, followed by a larger, bilateral R2 component. We investigated the circuitry that underlies this reflex in macaque (Macaca fascicularis and Macaca mulatta) monkeys by the use of single and dual tracer methods. Injection of retrograde tracer into the facial nucleus labeled neurons in the principal trigeminal nucleus, and in the spinal nucleus pars oralis and interpolaris, bilaterally, and in pars caudalis, ipsilaterally. Injection of anterograde tracer into the principal trigeminal nucleus labeled axons that directly terminated on ObOc motoneurons, with an ipsilateral predominance. Injection of anterograde tracer into pars caudalis of the spinal trigeminal nucleus labeled axons that directly terminated on ipsilateral ObOc motoneurons. The observed pattern of labeling indicates that the reticular formation ventromedial to the principal and spinal nuclei also contributes extensive bilateral input to ObOc motoneurons. Thus, much of the trigeminal sensory complex is in a position to supply a monosynaptic drive for lid closure, and the adjacent reticular formation can supply a disynaptic drive. These findings indicate that the assignment of the R1 and R2 components of the blink reflex to different parts of the trigeminal sensory complex cannot be exclusively based on subdivision connectional relationships with facial motoneurons. The characteristics of the R2 component may be due, instead, to other circuit properties.
Keywords: associative learning; classical conditioning; eyelid; facial nucleus; primate; somatosensory; trigeminal nucleus.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
Figures




Similar articles
-
Macaque monkey trigeminal blink reflex circuits targeting levator palpebrae superioris motoneurons.J Comp Neurol. 2021 Oct;529(14):3389-3409. doi: 10.1002/cne.25198. Epub 2021 Jun 11. J Comp Neurol. 2021. PMID: 34101199 Free PMC article.
-
Blink-related sensorimotor anatomy in the rat.Anat Embryol (Berl). 2003 Oct;207(3):193-208. doi: 10.1007/s00429-003-0341-6. Epub 2003 Oct 9. Anat Embryol (Berl). 2003. PMID: 14551765
-
Trigeminal inputs to eyeblink motoneurons in the rabbit.Exp Neurol. 1996 Dec;142(2):244-57. doi: 10.1006/exnr.1996.0195. Exp Neurol. 1996. PMID: 8934557
-
F wave, A wave, H reflex, and blink reflex.Handb Clin Neurol. 2019;160:225-239. doi: 10.1016/B978-0-444-64032-1.00015-1. Handb Clin Neurol. 2019. PMID: 31277850 Review.
-
Spontaneous, Voluntary, and Reflex Blinking in Clinical Practice.J Clin Neurophysiol. 2019 Nov;36(6):415-421. doi: 10.1097/WNP.0000000000000561. J Clin Neurophysiol. 2019. PMID: 31688324 Review.
Cited by
-
Short-latency prepulse inhibition of the trigeminal blink reflex.Front Neurosci. 2024 May 22;18:1357368. doi: 10.3389/fnins.2024.1357368. eCollection 2024. Front Neurosci. 2024. PMID: 38841093 Free PMC article.
-
Target Site of Prepulse Inhibition of the Trigeminal Blink Reflex in Humans.J Neurosci. 2023 Jan 11;43(2):261-269. doi: 10.1523/JNEUROSCI.1468-22.2022. Epub 2022 Nov 28. J Neurosci. 2023. PMID: 36443001 Free PMC article.
-
Macaque monkey trigeminal blink reflex circuits targeting levator palpebrae superioris motoneurons.J Comp Neurol. 2021 Oct;529(14):3389-3409. doi: 10.1002/cne.25198. Epub 2021 Jun 11. J Comp Neurol. 2021. PMID: 34101199 Free PMC article.
-
New reflexes during resting motor threshold determinations.Aust N Z J Psychiatry. 2023 Sep;57(9):1200-1201. doi: 10.1177/00048674231166891. Epub 2023 Apr 12. Aust N Z J Psychiatry. 2023. PMID: 37042302 Free PMC article. No abstract available.
-
A Novel Tectal/Pretectal Population of Premotor Lens Accommodation Neurons.Invest Ophthalmol Vis Sci. 2022 Jan 3;63(1):35. doi: 10.1167/iovs.63.1.35. Invest Ophthalmol Vis Sci. 2022. PMID: 35084433 Free PMC article.
References
-
- Barnerssoi M, May PJ (2016) Postembedding immunohistochemistry for inhibitory neurotransmitters in conjunction with neuroanatomical tracers. In: Transmission Electron Microscopy Methods for Understanding the Brain; Ed: Van Bockstaele EJ; Neuromethods; vol 115, pp 181–203.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

