Bortezomib suppresses self-renewal and leukemogenesis of leukemia stem cell by NF-ĸB-dependent inhibition of CDK6 in MLL-rearranged myeloid leukemia
- PMID: 33599085
- PMCID: PMC7957264
- DOI: 10.1111/jcmm.16377
Bortezomib suppresses self-renewal and leukemogenesis of leukemia stem cell by NF-ĸB-dependent inhibition of CDK6 in MLL-rearranged myeloid leukemia
Abstract
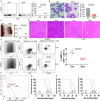
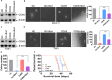
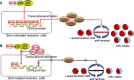
Acute myeloid leukaemia (AML) with chromosomal rearrangements involving the H3K4 methyltransferase mixed-lineage leukaemia (MLL) is an aggressive subtype with low overall survival. Bortezomib (Bort) is first applied in multiple myeloma. However, whether bort possesses anti-self-renewal and leukemogenesis of leukaemia stem cell (LSC) in AML with MLL rearrangements is still unclear. Here, we found that bort suppressed cell proliferation and decreased colony formation in human and murine leukaemic blasts. Besides, bort reduced the frequency and function of LSC, inhibited the progression, and extended the overall survival in MLL-AF9 (MF9) -transformed leukaemic mice. Furthermore, bort decreased the percentage of human LSC (CD34+ CD38- ) cells and extended the overall survival in AML blasts-xenografted NOD/SCID-IL2Rγ (NSG) mice. Mechanistically, cyclin dependent kinase 6 (CDK6) was identified as a bort target by RNA sequencing. Bort reduced the expressions of CDK6 by inhibiting NF ĸB recruitment to the promoter of CDK6, leading to the abolishment of NF ĸB DNA-binding activity for CDK6 promoter. Overexpression of CDK6 partially rescued bort-induced anti-leukemogenesis. Most importantly, bort had little side-effect against the normal haematological stem and progenitor cell (HSPC) and did not affect CDK6 expression in normal HSPC. In conclusion, our results suggest that bort selectively targets LSC in MLL rearrangements. Bort might be a prospective drug for AML patients bearing MLL rearrangements.
Keywords: Bortezomib; MLL rearrangements; cyclin dependent kinase 6; leukaemia stem cell.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem‐cell development. Nat Rev Cancer. 2007;7:823‐833. - PubMed
-
- Krivtsov AV, Twomey D, Feng Z, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL‐AF9. Nature. 2006;442:818‐822. - PubMed
-
- Kumar AR, Hudson WA, Chen W, et al. Hoxa9 influences the phenotype but not the incidence of Mll‐AF9 fusion gene leukemia. Blood. 2004;103:1823‐1828. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

