Regenerative potential of epicardium-derived extracellular vesicles mediated by conserved miRNA transfer
- PMID: 33599250
- PMCID: PMC8803084
- DOI: 10.1093/cvr/cvab054
Regenerative potential of epicardium-derived extracellular vesicles mediated by conserved miRNA transfer
Abstract
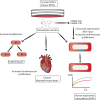
Aims: After a myocardial infarction, the adult human heart lacks sufficient regenerative capacity to restore lost tissue, leading to heart failure progression. Finding novel ways to reprogram adult cardiomyocytes into a regenerative state is a major therapeutic goal. The epicardium, the outermost layer of the heart, contributes cardiovascular cell types to the forming heart and is a source of trophic signals to promote heart muscle growth during embryonic development. The epicardium is also essential for heart regeneration in zebrafish and neonatal mice and can be reactivated after injury in adult hearts to improve outcome. A recently identified mechanism of cell-cell communication and signalling is that mediated by extracellular vesicles (EVs). Here, we aimed to investigate epicardial signalling via EV release in response to cardiac injury and as a means to optimize cardiac repair and regeneration.
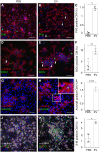
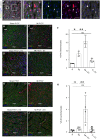
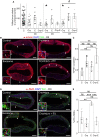
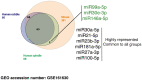
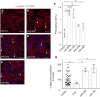
Methods and results: We isolated epicardial EVs from mouse and human sources and targeted the cardiomyocyte population. Epicardial EVs enhanced proliferation in H9C2 cells and in primary neonatal murine cardiomyocytes in vitro and promoted cell cycle re-entry when injected into the injured area of infarcted neonatal hearts. These EVs also enhanced regeneration in cryoinjured engineered human myocardium (EHM) as a novel model of human myocardial injury. Deep RNA-sequencing of epicardial EV cargo revealed conserved microRNAs (miRs) between human and mouse epicardial-derived exosomes, and the effects on cell cycle re-entry were recapitulated by administration of cargo miR-30a, miR-100, miR-27a, and miR-30e to human stem cell-derived cardiomyocytes and cryoinjured EHM constructs.
Conclusion: Here, we describe the first characterization of epicardial EV secretion, which can signal to promote proliferation of cardiomyocytes in infarcted mouse hearts and in a human model of myocardial injury, resulting in enhanced contractile function. Analysis of exosome cargo in mouse and human identified conserved pro-regenerative miRs, which in combination recapitulated the therapeutic effects of promoting cardiomyocyte proliferation.
Keywords: Epicardium; Extracellular vesicles; FUCCI; Human engineered myocardium; MicroRNA; Myocardial infarction; Regeneration.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures


References
-
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292. - PMC - PubMed
-
- Bollini S, Riley PR, Smart N.. Thymosin beta4: multiple functions in protection, repair and regeneration of the mammalian heart. Expert Opin Biol Ther 2015;15:163– 174. - PubMed
-
- Simoes FC, Riley PR.. The ontogeny, activation and function of the epicardium during heart development and regeneration. Development 2018;145:dev155994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

