On the emergence, spread and resistance of Candida auris: host, pathogen and environmental tipping points
- PMID: 33599604
- PMCID: PMC8346726
- DOI: 10.1099/jmm.0.001318
On the emergence, spread and resistance of Candida auris: host, pathogen and environmental tipping points
Abstract
Over a decade ago, a multidrug-resistant nosocomial fungus Candida auris emerged worldwide and has since become a significant challenge for clinicians and microbiologists across the globe. A resilient pathogen, C. auris survives harsh disinfectants, desiccation and high-saline environments. It readily colonizes the inanimate environment, susceptible patients and causes invasive infections that exact a high toll. Prone to misidentification by conventional microbiology techniques, C. auris rapidly acquires multiple genetic determinants that confer multidrug resistance. Whole-genome sequencing has identified four distinct clades of C. auris, and possibly a fifth one, in circulation. Even as our understanding of this formidable pathogen grows, the nearly simultaneous emergence of its distinct clades in different parts of the world, followed by their rapid global spread, remains largely unexplained. We contend that certain host-pathogen-environmental factors have been evolving along adverse trajectories for the last few decades, especially in regions where C. auris originally appeared, until these factors possibly reached a tipping point to compel the evolution, emergence and spread of C. auris. Comparative genomics has helped identify several resistance mechanisms in C. auris that are analogous to those seen in other Candida species, but they fail to fully explain how high-level resistance rapidly develops in this yeast. A better understanding of these unresolved aspects is essential not only for the effective management of C. auris patients, hospital outbreaks and its global spread but also for forecasting and tackling novel resistant pathogens that might emerge in the future. In this review, we discuss the emergence, spread and resistance of C. auris, and propose future investigations to tackle this resilient pathogen.
Keywords: Candida auris; antibiotics; antifungals; emergence; resistance.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


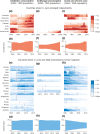
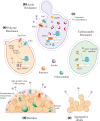
References
-
- CDC Infection Prevention and Control for Candida auris. Centers for Disease Control and Prevention; Atlanta, GA, USA. 2020. https://www.cdc.gov/fungal/candida-auris/c-auris-infection-control.html [accessed 20 Aug 2020].
-
- CDC Tracking Candida auris. Centers for Disease Control and Prevention; Atlanta, GA, USA. https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html [accessed 01 Dec 2020]. 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

