Virus infection of the CNS disrupts the immune-neural-synaptic axis via induction of pleiotropic gene regulation of host responses
- PMID: 33599611
- PMCID: PMC7891934
- DOI: 10.7554/eLife.62273
Virus infection of the CNS disrupts the immune-neural-synaptic axis via induction of pleiotropic gene regulation of host responses
Abstract
Treatment for many viral infections of the central nervous system (CNS) remains only supportive. Here we address a remaining gap in our knowledge regarding how the CNS and immune systems interact during viral infection. By examining the regulation of the immune and nervous system processes in a nonhuman primate model of West Nile virus neurological disease, we show that virus infection disrupts the homeostasis of the immune-neural-synaptic axis via induction of pleiotropic genes with distinct functions in each component of the axis. This pleiotropic gene regulation suggests an unintended off-target negative impact of virus-induced host immune responses on the neurotransmission, which may be a common feature of various viral infections of the CNS.
Keywords: functional genomics; immune system; immunology; infectious disease; inflammation; microbiology; neuronal system; pleiotropic genes; rhesus macaque; synapses; virus.
Conflict of interest statement
OM, DS, JK, KK, YX, MM, IM, JT, CM, JC, AP No competing interests declared
Figures

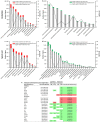






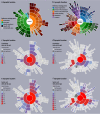

Similar articles
-
Genetic diversity in the collaborative cross model recapitulates human West Nile virus disease outcomes.mBio. 2015 May 5;6(3):e00493-15. doi: 10.1128/mBio.00493-15. mBio. 2015. PMID: 25944860 Free PMC article.
-
Immunological headgear: antiviral immune responses protect against neuroinvasive West Nile virus.Trends Mol Med. 2008 Jul;14(7):286-94. doi: 10.1016/j.molmed.2008.05.004. Epub 2008 Jun 6. Trends Mol Med. 2008. PMID: 18539532 Review.
-
Intrinsic Innate Immune Responses Control Viral Growth and Protect against Neuronal Death in an Ex Vivo Model of West Nile Virus-Induced Central Nervous System Disease.J Virol. 2021 Aug 25;95(18):e0083521. doi: 10.1128/JVI.00835-21. Epub 2021 Aug 25. J Virol. 2021. PMID: 34190599 Free PMC article.
-
Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion.J Virol. 2006 Oct;80(19):9349-60. doi: 10.1128/JVI.01122-06. J Virol. 2006. PMID: 16973541 Free PMC article. Review. No abstract available.
-
Interferon regulatory factor 5-dependent immune responses in the draining lymph node protect against West Nile virus infection.J Virol. 2014 Oct;88(19):11007-21. doi: 10.1128/JVI.01545-14. Epub 2014 Jul 16. J Virol. 2014. PMID: 25031348 Free PMC article.
Cited by
-
Pathogenesis and outcome of VA1 astrovirus infection in the human brain are defined by disruption of neural functions and imbalanced host immune responses.PLoS Pathog. 2023 Aug 18;19(8):e1011544. doi: 10.1371/journal.ppat.1011544. eCollection 2023 Aug. PLoS Pathog. 2023. PMID: 37595007 Free PMC article.
-
Inflammatory Response Associated with West Nile Neuroinvasive Disease: A Systematic Review.Viruses. 2024 Feb 29;16(3):383. doi: 10.3390/v16030383. Viruses. 2024. PMID: 38543749 Free PMC article.
-
Breeding honey bees (Apis mellifera L.) for low and high Varroa destructor population growth: Gene expression of bees performing grooming behavior.Front Insect Sci. 2023 Mar 6;3:951447. doi: 10.3389/finsc.2023.951447. eCollection 2023. Front Insect Sci. 2023. PMID: 38469529 Free PMC article.
-
Pathological features of West Nile and Usutu virus natural infections in wild and domestic animals and in humans: A comparative review.One Health. 2023 Mar 10;16:100525. doi: 10.1016/j.onehlt.2023.100525. eCollection 2023 Jun. One Health. 2023. PMID: 37363223 Free PMC article.
-
Monkeypox virus spreads from cell-to-cell and leads to neuronal death in human neural organoids.Nat Commun. 2025 Jun 30;16(1):5376. doi: 10.1038/s41467-025-61134-0. Nat Commun. 2025. PMID: 40588500 Free PMC article.
References
-
- Armah HB, Wang G, Omalu BI, Tesh RB, Gyure KA, Chute DJ, Smith RD, Dulai P, Vinters HV, Kleinschmidt-DeMasters BK, Wiley CA. Systemic distribution of west nile virus infection: postmortem immunohistochemical study of six cases. Brain Pathology. 2007;17:354–362. doi: 10.1111/j.1750-3639.2007.00080.x. - DOI - PMC - PubMed
-
- Betsem E, Kaidarova Z, Stramer SL, Shaz B, Sayers M, LeParc G, Custer B, Busch MP, Murphy EL. Correlation of west nile virus incidence in donated blood with west nile neuroinvasive disease rates, united states, 2010-2012. Emerging Infectious Diseases. 2017;23:212–219. doi: 10.3201/eid2302.161058. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

