Phosphate has dual roles in cross-bridge kinetics in rabbit psoas single myofibrils
- PMID: 33599680
- PMCID: PMC7885270
- DOI: 10.1085/jgp.202012755
Phosphate has dual roles in cross-bridge kinetics in rabbit psoas single myofibrils
Abstract
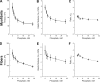
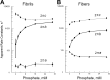
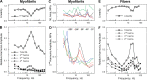
In this study, we aimed to study the role of inorganic phosphate (Pi) in the production of oscillatory work and cross-bridge (CB) kinetics of striated muscle. We applied small-amplitude sinusoidal length oscillations to rabbit psoas single myofibrils and muscle fibers, and the resulting force responses were analyzed during maximal Ca2+ activation (pCa 4.65) at 15°C. Three exponential processes, A, B, and C, were identified from the tension transients, which were studied as functions of Pi concentration ([Pi]). In myofibrils, we found that process C, corresponding to phase 2 of step analysis during isometric contraction, is almost a perfect single exponential function compared with skinned fibers, which exhibit distributed rate constants, as described previously. The [Pi] dependence of the apparent rate constants 2πb and 2πc, and that of isometric tension, was studied to characterize the force generation and Pi release steps in the CB cycle, as well as the inhibitory effect of Pi. In contrast to skinned fibers, Pi does not accumulate in the core of myofibrils, allowing sinusoidal analysis to be performed nearly at [Pi] = 0. Process B disappeared as [Pi] approached 0 mM in myofibrils, indicating the significance of the role of Pi rebinding to CBs in the production of oscillatory work (process B). Our results also suggest that Pi competitively inhibits ATP binding to CBs, with an inhibitory dissociation constant of ∼2.6 mM. Finally, we found that the sinusoidal waveform of tension is mostly distorted by second harmonics and that this distortion is closely correlated with production of oscillatory work, indicating that the mechanism of generating force is intrinsically nonlinear. A nonlinear force generation mechanism suggests that the length-dependent intrinsic rate constant is asymmetric upon stretch and release and that there may be a ratchet mechanism involved in the CB cycle.
© 2021 Kawai et al.
Figures








Similar articles
-
Oscillatory work and the step that generates force in single myofibrils from rabbit psoas.Pflugers Arch. 2024 Jun;476(6):949-962. doi: 10.1007/s00424-024-02935-y. Epub 2024 Apr 1. Pflugers Arch. 2024. PMID: 38558187
-
Correlation between cross-bridge kinetics obtained from Trp fluorescence of myofibril suspensions and mechanical studies of single muscle fibers in rabbit psoas.J Muscle Res Cell Motil. 2011 Dec;32(4-5):315-26. doi: 10.1007/s10974-011-9264-7. Epub 2011 Oct 18. J Muscle Res Cell Motil. 2011. PMID: 22006015
-
ATP binding and cross-bridge detachment steps during full Ca²⁺ activation: comparison of myofibril and muscle fibre mechanics by sinusoidal analysis.J Physiol. 2012 Jul 15;590(14):3361-73. doi: 10.1113/jphysiol.2012.228379. Epub 2012 May 14. J Physiol. 2012. PMID: 22586213 Free PMC article.
-
Kinetic coupling of phosphate release, force generation and rate-limiting steps in the cross-bridge cycle.J Muscle Res Cell Motil. 2017 Aug;38(3-4):275-289. doi: 10.1007/s10974-017-9482-8. Epub 2017 Sep 16. J Muscle Res Cell Motil. 2017. PMID: 28918606 Review.
-
The cross-bridge cycle and skeletal muscle fatigue.J Appl Physiol (1985). 2008 Feb;104(2):551-8. doi: 10.1152/japplphysiol.01200.2007. Epub 2007 Dec 27. J Appl Physiol (1985). 2008. PMID: 18162480 Review.
Cited by
-
Theoretical treatment of tension transients in muscle following sudden changes in orthophosphate concentration: implications for energy transduction.J Muscle Res Cell Motil. 2025 Jul 14. doi: 10.1007/s10974-025-09698-8. Online ahead of print. J Muscle Res Cell Motil. 2025. PMID: 40658284
-
The effect of gender and obesity in modulating cross-bridge function in cardiac muscle fibers.J Muscle Res Cell Motil. 2022 Dec;43(4):157-172. doi: 10.1007/s10974-022-09627-z. Epub 2022 Aug 22. J Muscle Res Cell Motil. 2022. PMID: 35994221
-
The elementary step that generates force and sinusoidal analysis in striated muscle fibers.J Muscle Res Cell Motil. 2025 Jun;46(2):83-118. doi: 10.1007/s10974-025-09693-z. Epub 2025 Jul 7. J Muscle Res Cell Motil. 2025. PMID: 40622514 Free PMC article. Review.
-
Toward an understanding of myofibrillar function in health and disease.J Gen Physiol. 2021 Mar 1;153(3):e202112880. doi: 10.1085/jgp.202112880. J Gen Physiol. 2021. PMID: 33620422 Free PMC article.
References
-
- Abbott, R.H. 1973. An interpretation of the effects of fiber length and calcium on the mechanical properties of insect flight muscle. Cold Spring Harb. Symp. Quant. Biol. 37:647–654. 10.1101/SQB.1973.037.01.078 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

