Response Rates to Anti-PD-1 Immunotherapy in Microsatellite-Stable Solid Tumors With 10 or More Mutations per Megabase
- PMID: 33599686
- PMCID: PMC7893543
- DOI: 10.1001/jamaoncol.2020.7684
Response Rates to Anti-PD-1 Immunotherapy in Microsatellite-Stable Solid Tumors With 10 or More Mutations per Megabase
Abstract
Importance: In June 2020, the US Food and Drug Administration approved the anti-programmed cell death 1 drug pembrolizumab for patients with malignant solid tumors of any histologic type with high tumor mutational burden (TMB; ≥10 mutations per megabase). The predictive value of this universal cutoff for high TMB is not well understood.
Objective: To examine the performance of a universal definition of high TMB in an independent cohort of patients with solid tumors treated with immune checkpoint inhibitors.
Design, setting, and participants: This retrospective cohort study included 1678 patients at a single cancer referral center treated with immune checkpoint inhibitors from January 1, 2015, to December 31, 2018. Patients had 16 different cancer types and were treated with anti-programmed cell death 1 or programmed cell death ligand-1 immunotherapy. Tumors underwent next-generation sequencing.
Exposures: At least 1 dose of immune checkpoint inhibitors.
Main outcomes and measures: Best overall response to immune checkpoint inhibitor therapy. The hypothesis tested was formulated after data collection and prior to analysis.
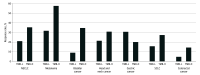
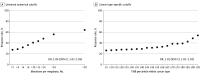
Results: Of 1678 patients, 924 (55%) were male, and the median age was 64 years (interquartile range, 55-71 years). Using the universal cutoff of 10 mutations per megabase, 416 tumors (25%) were categorized as having high TMB. Across cancer types, the proportion of TMB-high tumors ranged from 0% of kidney cancers to 53% of melanomas (113 of 214). Tumors categorized as TMB-high had higher response rates compared with TMB-low tumors in only 11 of 16 cancer types. In the entire cohort, response rates increased with higher cutoffs for TMB-high categorization, reaching 41% (169 of 416) for TMB more than 10 and 56% (90 of 161) for TMB more than 18, the highest TMB decile. Response rates also increased with TMB percentile within cancer type. Using cancer-specific cutoffs, 457 tumors (27%) were categorized as TMB-high. Response rates within cancer type ranged from 4% for pancreatic cancer (1 of 26) to 70% for melanoma (46 of 66). Cancer-specific cutoffs were associated with numerically higher response rates for TMB-high compared with TMB-low tumors in 14 of 16 cancer types.
Conclusions and relevance: The data from this cohort study validate the finding of generally higher response rates following immune checkpoint inhibitor therapy for tumors with TMB of 10 or more mutations per megabase, across multiple cancer types. However, the predictive value of a universal numerical threshold for TMB-high was limited, owing to variability across cancer types and unclear associations with survival outcomes. Further investigation will help define cancer type-specific TMB cutoffs to guide decision-making.
Conflict of interest statement
Figures
Comment in
-
Inappropriate Use of the Same Cutoff by Different Sequencing Panels for Tumor Mutation Burden as Immunotherapy Biomarker-Reply.JAMA Oncol. 2021 Aug 1;7(8):1245-1246. doi: 10.1001/jamaoncol.2021.1870. JAMA Oncol. 2021. PMID: 34110358 No abstract available.
-
Inappropriate Use of the Same Cutoff by Different Sequencing Panels for Tumor Mutation Burden as Immunotherapy Biomarker.JAMA Oncol. 2021 Aug 1;7(8):1244-1245. doi: 10.1001/jamaoncol.2021.1867. JAMA Oncol. 2021. PMID: 34110373 No abstract available.
References
-
- US Food and Drug Administration . FDA approves pembrolizumab for adults and children with TMB-H solid tumors. Published June 17, 2020. Accessed July 17, 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pemb...
-
- US National Library of Medicine . Study of pembrolizumab (MK-3475) in participants with advanced solid tumors (MK-3475-158/KEYNOTE-158). ClinicalTrials.gov. Accessed July 17, 2020. https://clinicaltrials.gov/ct2/show/NCT02628067
-
- Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353-1365. doi: 10.1016/S1470-2045(20)30445-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

