Long-term Safety and Efficacy of the Anti-MAdCAM-1 Monoclonal Antibody Ontamalimab [SHP647] for the Treatment of Ulcerative Colitis: The Open-label Study TURANDOT II
- PMID: 33599720
- PMCID: PMC8218706
- DOI: 10.1093/ecco-jcc/jjab023
Long-term Safety and Efficacy of the Anti-MAdCAM-1 Monoclonal Antibody Ontamalimab [SHP647] for the Treatment of Ulcerative Colitis: The Open-label Study TURANDOT II
Abstract
Background and aims: Ontamalimab, a fully-human monoclonal antibody targeting MAdCAM-1, induced remission in patients with moderate-to-severe ulcerative colitis [UC] in the TURANDOT study. We aimed to assess long-term safety, tolerability, and efficacy of ontamalimab in TURANDOT II.
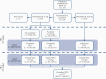
Methods: TURANDOT II was a phase 2, multicentre, open-label [OL] study in patients with moderate-to-severe UC who completed TURANDOT on placebo or ontamalimab (NCT01771809). Patients were randomised to 75 mg or 225 mg ontamalimab every 4 weeks for 72 weeks [OL1]. The dosage could be increased to 225 mg from Week 8 at the investigator's discretion. All patients then received 75 mg every 4 weeks for 72 weeks [OL2], followed by 6-month safety follow-up. The primary objective was safety, measured by adverse events [AEs], serious AEs [SAEs], and AEs leading to withdrawal. Mucosal healing [MH; centrally read endoscopy] was assessed.
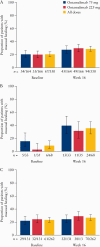
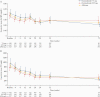
Results: Of 330 patients, 180 completed OL1; 94 escalated to 225 mg; 127 completed OL2. Overall, 36.1% experienced drug-related AEs. The most common SAE [10.0%] was worsening/ongoing UC; 5.5% of patients had serious infections, the most common being gastroenteritis [0.9%]. One death and four cancers [all unrelated to ontamalimab] occurred. No PML [progressive multifocal leukoencephalopathy]/lymphoproliferative disorders occurred. Geometric mean high-sensitivity C-reactive protein [hsCRP] and faecal calprotectin decreased across OL1 in both dose groups. The proportion of patients assigned to placebo in TURANDOT achieving MH increased from 8.8% [6/68] at baseline to 35.3% at Week 16 [24/68; non-responder imputation]. The corresponding increase in the ontamalimab group was from 23.3% [61/262] to 26.7% [70/262].
Conclusions: Ontamalimab was well tolerated up to 144 weeks in patients with moderate-to-severe UC, with good safety and efficacy.
Keywords: MAdCAM-1; Ulcerative colitis; phase 2.
© The Author(s) 2021. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Figures


References
-
- Paschos P, Katsoula A, Salanti G, Giouleme O, Athanasiadou E, Tsapas A. Systematic review with network meta-analysis: the impact of medical interventions for moderate-to-severe ulcerative colitis on health-related quality of life. Aliment Pharmacol Ther 2018;48:1174–85. - PubMed
-
- Peyrin-Biroulet L, Sandborn W, Sands BE, et al. . Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE]: determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–38. - PubMed
-
- Dubinsky MC. Reviewing treatments and outcomes in the evolving landscape of ulcerative colitis. Postgrad Med 2017;129:538–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

