Multisystem inflammatory syndrome in children related to COVID-19: a systematic review
- PMID: 33599835
- PMCID: PMC7890544
- DOI: 10.1007/s00431-021-03993-5
Multisystem inflammatory syndrome in children related to COVID-19: a systematic review
Abstract
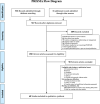
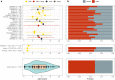
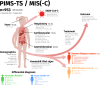
An association between a novel pediatric hyperinflammatory condition and SARS-CoV-2 was recently published and termed pediatric inflammatory multisystem syndrome, temporally associated with SARS-CoV-2 (PIMS-TS) or multisystem inflammatory syndrome (in children) (MIS(-C)). We performed a systematic review and describe the epidemiological, clinical, and prognostic characteristics of 953 PIMS-TS/MIS(-C) cases in 68 records. Additionally, we studied the sensitivity of different case definitions that are currently applied. PIMS-TS/MIS(-C) presents at a median age of 8 years. Epidemiological enrichment for males (58.9%) and ethnic minorities (37.0% Black) is present. Apart from obesity (25.3%), comorbidities are rare. PIMS-TS/MIS(-C) is characterized by fever (99.4%), gastrointestinal (85.6%) and cardiocirculatory manifestations (79.3%), and increased inflammatory biomarkers. Nevertheless, 50.3% present respiratory symptoms as well. Over half of patients (56.3%) present with shock. The majority of the patients (73.3%) need intensive care treatment, including extracorporal membrane oxygenation (ECMO) in 3.8%. Despite severe disease, mortality is rather low (1.9%). Of the currently used case definitions, the WHO definition is preferred, as it is more precise, while encompassing most cases.Conclusion: PIMS-TS/MIS(-C) is a severe, heterogeneous disease with epidemiological enrichment for males, adolescents, and racial and ethnic minorities. However, mortality rate is low and short-term outcome favorable. Long-term follow-up of chronic complications and additional clinical research to elucidate the underlying pathogenesis is crucial. What is Known: • A novel pediatric inflammatory syndrome with multisystem involvement has been described in association with SARS-CoV-2. • To date, the scattered reporting of cases and use of different case definitions provides insufficient insight in the full clinical spectrum, epidemiological and immunological features, and prognosis. What is New: • This systematic review illustrates the heterogeneous spectrum of PIMS-TS/MIS(-C) and its epidemiological enrichment for males, adolescents, and racial and ethnic minorities. • Despite its severe presentation, overall short-term outcome is good. • The WHO MIS definition is preferred, as it is more precise, while encompassing most cases.
Keywords: COVID-19; MIS-C; PIMS-TS; SARS-CoV-2.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- WHO (2020) Novel coronavirus – China (2020, Jan 12). http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/. Accessed 23 Jul 2020
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J', Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

