Fluid restriction in the management of transient tachypnea of the newborn
- PMID: 33599990
- PMCID: PMC8094737
- DOI: 10.1002/14651858.CD011466.pub2
Fluid restriction in the management of transient tachypnea of the newborn
Abstract
Background: Transient tachypnea of the newborn (TTN) is caused by delayed clearance of lung fluid at birth. TTN typically appears within the first two hours of life in term and late preterm neonates and is characterized by tachypnea and signs of respiratory distress. Although it is usually a self-limited condition, admission to a neonatal unit is frequently required for monitoring and providing respiratory support. Restricting intake of fluids administered to these infants in the first days of life might improve clearance of lung liquid, thus reducing the effort required to breathe, improving respiratory distress, and potentially reducing the duration of tachypnea.
Objectives: To evaluate the efficacy and safety of restricted fluid therapy as compared to standard fluid therapy in decreasing the duration of oxygen administration and the need for noninvasive or invasive ventilation among neonates with TTN.
Search methods: We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 12), in the Cochrane Library; Ovid MEDLINE and electronic ahead of print publications, in-process & other non-indexed citations, Daily and Versions(R); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL), on December 6, 2019. We also searched clinical trial databases and the reference lists of retrieved articles for randomized controlled trials and quasi-randomized trials.
Selection criteria: We included randomized controlled trials (RCTs), quasi-RCTs, and cluster trials on fluid restriction in term and preterm neonates with the diagnosis of TTN or delayed adaptation during the first week after birth.
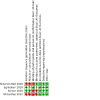
Data collection and analysis: For each of the included trials, two review authors independently extracted data (e.g. number of participants, birth weight, gestational age, duration of oxygen therapy, need for continuous positive airway pressure [CPAP], need for mechanical ventilation, duration of mechanical ventilation) and assessed the risk of bias (e.g. adequacy of randomization, blinding, completeness of follow-up). The primary outcome considered in this review was the duration of supplemental oxygen therapy in hours or days. We used the GRADE approach to assess the certainty of evidence.
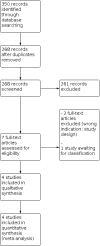
Main results: Four trials enrolling 317 infants met the inclusion criteria. Three trials enrolled late preterm and term infants with TTN, and the fourth trial enrolled only term infants with TTN. Infants were on various methods of respiratory support at the time of enrollment including room air, oxygen, or nasal CPAP. Infants in the fluid-restricted group received 15 to 20 mL/kg/d less fluid than those in the control group for varying durations after enrollment. Two studies had high risk of selection bias, and three out of four had high risk of performance bias. Only one study had low risk of detection bias, with two at high risk and one at unclear risk. The certainty of evidence for all outcomes was very low due to imprecision of estimates and unclear risk of bias. Two trials reported the primary duration of supplemental oxygen therapy. We are uncertain whether fluid restriction decreases or increases the duration of supplemental oxygen therapy (mean difference [MD] -12.95 hours, 95% confidence interval [CI] -32.82 to 6.92; I² = 98%; 172 infants). Similarly, there is uncertainty for various secondary outcomes including incidence of hypernatremia (serum sodium > 145 mEq/L, risk ratio [RR] 4.0, 95% CI 0.46 to 34.54; test of heterogeneity not applicable; 1 trial, 100 infants), hypoglycemia (blood glucose < 40 mg/dL, RR 1.0, 95% CI 0.15 to 6.82; test of heterogeneity not applicable; 2 trials, 164 infants), endotracheal ventilation (RR 0.73, 95% CI 0.24 to 2.23; I² = 0%; 3 trials, 242 infants), need for noninvasive ventilation (RR 0.40, 95% CI 0.14 to 1.17; test of heterogeneity not applicable; 2 trials, 150 infants), length of hospital stay (MD -0.92 days, 95% CI -1.53 to -0.31; test of heterogeneity not applicable; 1 trial, 80 infants), and cumulative weight loss at 72 hours of age (%) (MD 0.24, 95% CI -1.60 to 2.08; I² = 89%; 2 trials, 156 infants). We did not identify any ongoing trials; however, one trial is awaiting classification.
Authors' conclusions: We found limited evidence to establish the benefits and harms of fluid restriction in the management of TTN. Given the very low certainty of available evidence, it is impossible to determine whether fluid restriction is safe or effective for management of TTN. However, given the simplicity of the intervention, a well-designed trial is justified.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
NG has no interests to declare.
MB has received research funding from an ALF grant (non‐profit ‐ Lund University) and the Crafoord Foundation (non‐profit) for research projects not related to Cochrane.
DC has no interests to declare.
Figures












Update of
References
References to studies included in this review
Akbarian Rad 2018 {published data only}
-
- Akbarian Rad Z, Gorji Rad M, Haghshenas M. Effects of restrictive fluid management in transient tachypnea in neonates. Iranian Journal of Neonatology 2018;9(4):47-52. [DOI: 10.22038/ijn.2018.27719.1371] - DOI
Eghbalian 2018 {published data only}
-
- Eghbalian F, Sabzehei M, Emamzadeh N, Shokouhi M, Basiri B, Faradmal J, et al. Comparison of restricted fluid volume with standard fluid volume in management of transient tachypnea of the newborns: a randomized controlled trial. International Journal of Pediatrics 2018;6(9):8289-96. [DOI: 10.22038/ijp.2018.30462.2677] - DOI
-
- IRCT20120925010933N10. Effect of fluid therapy on transient tachypnea of newborn [Comparison of restricted fluid volume with standard fluid volume In management of transient tachypnea of newborn]. www.irct.ir/trial/29154 (first received February 1, 2018).
Sardar 2020 {unpublished data only}
-
- CTRI/2019/01/017125. A study comparing liberal versus restricted fluid administration in newborn with respiratory distress after birth. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=29222&EncHid=&u... (first received January 17, 2019).
-
- Sardar S, Pal S, Mishra R. A randomized controlled trial of restricted versus standard fluid management in late preterm and term infants with transient tachypnea of the newborn. Journal of Neonatal-Perinatal Medicine 2020;13(4):Online ahead of print. [DOI: DOI: 10.3233/NPM-190400] [PMID: ] - PubMed
Stroustrup 2012 {published data only}
-
- Stroustrup A, Holzman IR, MISC2. Prospective randomized controlled trial of restrictive fluid management in transient tachypnea of the newborn. Clinical and Translational Science Conference: 2011 Clinical and Translational Research and Education Meeting: ACRT/AFMR/SCTS Joint Annual Meeting, Washington, DC, USA. April 2011:105.
-
- Stroustrup A, Holzman IR. Prospective randomized controlled trial of restrictive fluid management in transient tachypnea of the newborn. In: docplayer.net/6751965-Program-guide-23rd-annual-meeting-march-25-27-2011.... 2011. - PMC - PubMed
-
- Stroustrup A, Trasande L, Holzman IR. Less Is more: cost savings of fluid restriction in transient tachypnea of the newborn. In: docplayer.net/6751965-Program-guide-23rd-annual-meeting-march-25-27-2011.... 2011.
References to studies excluded from this review
CTRI/2019/04/018661 {published data only}
-
- CTRI/2019/04/018661. Measuring the specific gravity of urine for helping in assessment of how much intravenous fluids are required in newborns admitted to the neonatal intensive care unit. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=32643&EncHid=&u... (first received April 18, 2019).
References to studies awaiting assessment
Stroustrup 2010 {published data only}
-
- Stroustrup A, Trasande L, Holzman IR. Prospective randomized control trial of restrictive fluid management in transient tachypnea of the newborn. In: www.abstracts2view.com/pas/. Vol. 814. 2010. - PMC - PubMed
Additional references
Barker 2002
Bell 2014
Bland 1978
Clark 2005
Demling 1978
Egger 1997
Gowen 1988
-
- Gowen CW Jr, Lawson EE, Gingras J, Boucher RC, Gatzy JT, Knowles MR. Electrical potential difference and ion transport across nasal epithelium of term neonates: correlation with mode of delivery, transient tachypnea of the newborn, and respiratory rate. Journal of Pediatrics 1988;113(1 Pt 1):121-7. [DOI: 10.1016/s0022-3476(88)80545-6] [PMID: ] - DOI - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 26 June 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), Accessed June 26, 2020. Available at gradepro.org.
Greenough 1992
Hibbard 2010
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. - PMC - PubMed
Irestedt 1982
-
- Irestedt L, Lagercrantz H, Hjemdahl P, Hagnevik K, Belfrage P. Fetal and maternal plasma catecholamine levels at elective cesarean section under general or epidural anesthesia versus vaginal delivery. American Journal of Obstetrics and Gynecology 1982;142(8):1004-10. [DOI: 10.1016/0002-9378(82)90783-9] [PMID: ] - DOI - PubMed
Jain 2006
Jefferies 2013
Kao 1983
Kao 2008
Karabayir 2006
Kasab 2015
Kumar 1996
Lorenz 1995
-
- Lorenz JM, Kleinman LI, Ahmed G, Markarian K. Phases of fluid and electrolyte homeostasis in the extremely low birth weight infant. Pediatrics 1995;96(3 Pt 1):484-9. [PMID: ] - PubMed
Lorenz 2002
-
- Lorenz JM. Fluid and electrolyte therapy in the newborn infant. In: Burg FD, Polin RA, Ingelfinger JR, Gershon A, editors(s). Current Pediatric Therapy. 17th edition. Philadelphia: WB Saunders, 2002:29-35.
Martin 2010
-
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Osterman MJ. Births: final data for 2008. National Vital Statistics Reports 2010;59(1):1, 3-71. [PMID: ] - PubMed
Oh 2005
-
- Oh W, Poindexter BB, Perritt R, Lemons JA, Bauer CR, Ehrenkranz RA, et al. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Journal of Pediatrics 2005;147(6):786-90. [DOI: 10.1016/j.jpeds.2005.06.039] [PMID: ] - DOI - PubMed
Ramachandrappa 2008
Rawlings 1984
RevMan 2020 [Computer program]
-
- The Cochrane Collaboration Review Manager (RevMan). Version 5.4. The Cochrane Collaboration, 2020.
Riskin 2005
Rubaltelli 1998
-
- Rubaltelli FF, Bonafe L, Tangucci M, Spagnolo A, Dani C. Epidemiology of neonatal acute respiratory disorders. A multicenter study on incidence and fatality rates of neonatal acute respiratory disorders according to gestational age, maternal age, pregnancy complications and type of delivery. Italian Group of Neonatal Pneumology. Biology of the Neonate 1998;74(1):7-15. [DOI: 10.1159/000014005] [PMID: ] - DOI - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shaffer 1987a
Shaffer 1987b
-
- Shaffer SG, Quimiro CL, Anderson JV, Hall RT. Postnatal weight changes in low birth weight infants. Pediatrics 1987;79(5):702-5. [PMID: ] - PubMed
Silverman 1956
-
- Silverman WA, Andersen DH. A controlled clinical trial of effects of water mist on obstructive respiratory signs, death rate and necropsy findings among premature infants. Pediatrics 1956;17(1):1-10. [PMID: ] - PubMed
Tutdibi 2010
Wiswell 1985
-
- Wiswell TE, Rawlings JS, Smith FR, Goo ED. Effect of furosemide on the clinical course of transient tachypnea of the newborn. Pediatrics 1985;75(5):908-10. [PMID: ] - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

