Chemoenzymatic Total Synthesis of Natural Products
- PMID: 33600149
- PMCID: PMC8210581
- DOI: 10.1021/acs.accounts.0c00810
Chemoenzymatic Total Synthesis of Natural Products
Abstract
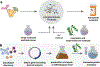
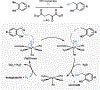
The total synthesis of structurally complex natural products has challenged and inspired generations of chemists and remains an exciting area of active research. Despite their history as privileged bioactivity-rich scaffolds, the use of natural products in drug discovery has waned. This shift is driven by their relatively low abundance hindering isolation from natural sources and the challenges presented by their synthesis. Recent developments in biocatalysis have resulted in the application of enzymes for the construction of complex molecules. From the inception of the Narayan lab in 2015, we have focused on harnessing the exquisite selectivity of enzymes alongside contemporary small molecule-based approaches to enable concise chemoenzymatic routes to natural products.We have focused on enzymes from various families that perform selective oxidation reactions. For example, we have targeted xyloketal natural products through a strategy that relies on a chemo- and site-selective biocatalytic hydroxylation. Members of the xyloketal family are characterized by polycyclic ketal cores and demonstrate potent neurological activity. We envisioned assembling a representative xyloketal natural product (xyloketal D) involving a biocatalytically generated ortho-quinone methide intermediate. The non-heme iron (NHI) dependent monooxygenase ClaD was used to perform the benzylic hydroxylation of a resorcinol precursor, the product of which can undergo spontaneous loss of water to form an ortho-quinone methide under mild conditions. This intermediate was trapped using a chiral dienophile to complete the total synthesis of xyloketal D.A second class of biocatalytic oxidation that we have employed in synthesis is the hydroxylative dearomatization of resorcinol compounds using flavin-dependent monooxygenases (FDMOs). We anticipated that the catalyst-controlled site- and stereoselectivity of FDMOs would enable the total synthesis of azaphilone natural products. Azaphilones are bioactive compounds characterized by a pyranoquinone bicyclic core and a fully substituted chiral carbon atom. We leveraged the stereodivergent reactivity of FDMOs AzaH and AfoD to achieve the enantioselective synthesis of trichoflectin enantiomers, deflectin 1a, and lunatoic acid. We also leveraged FDMOs to construct tropolone and sorbicillinoid natural products. Tropolones are a structurally diverse class of bioactive molecules characterized by an aromatic cycloheptatriene core bearing an α-hydroxyketone moiety. We developed a two-step biocatalytic cascade to the tropolone natural product stipitatic aldehyde using the FDMO TropB and a NHI monooxygenase TropC. The FDMO SorbC obtained from the sorbicillin biosynthetic pathway was used in the concise total synthesis of a urea sorbicillinoid natural product.Our long-standing interest in using enzymes to carry out C-H hydroxylation reactions has also been channeled for the late-stage diversification of complex scaffolds. For example, we have used Rieske oxygenases to hydroxylate the tricyclic core common to paralytic shellfish toxins. The systemic toxicity of these compounds can be reduced by adding hydroxyl and sulfate groups, which improves their properties and potential as therapeutic agents. The enzymes SxtT, GxtA, SxtN, and SxtSUL were used to carry out selective C-H hydroxylation and O-sulfation in saxitoxin and related structures. We conclude this Account with a discussion of existing challenges in biocatalysis and ways we can currently address them.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
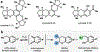

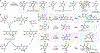



References
-
- Doyon TJ; Perkins JC; Baker Dockrey SA; Romero EO; Skinner KC; Zimmerman PM; Narayan ARH Chemoenzymatic o-Quinone Methide Formation. J. Am. Chem. Soc 2019, 141, 20269–20277. - PMC - PubMed
-
This study highlights the use of o-quinone methides generated through biocatalytic oxidation in the chemoenzymatic total synthesis of xyloketal natural products.
-
- Pyser JB; Baker Dockrey SA; Rodríguez Benítez A; Joyce LA; Wiscons RA; Smith JL; Narayan ARH Stereodivergent, Chemoenzymatic Synthesis of Azaphilone Natural Products. J. Am. Chem. Soc 2019, 141, 18551–18559 - PMC - PubMed
-
This study highlights the use of sequence similarity network (SSN) analysis to identify stereodivergent biocatalysts and their applications in the chemoenzymatic total synthesis of azaphilone natural products.
-
- Lukowski AL; Denomme N; Hinze ME; Hall S; Isom LL; Narayan ARH Biocatalytic Detoxification of Paralytic Shellfish Toxins. ACS Chem. Biol 2019, 14, 941–948. - PMC - PubMed
-
This study highlights the use of enzymes used for carrying out selective C-H hydroxylation and sulfation of paralytic shellfish toxins.
-
- Baker Dockrey SA; Lukowski AL; Becker MR; Narayan ARH Biocatalytic site- and enantioselective oxidative dearomatization of phenols. Nat. Chem 2018, 10, 119–125. - PMC - PubMed
-
This study highlights the use of enzymes derived from natural product biosynthetic pathways in the chemoenzymatic total synthesis of azaphilone, tropolone, and sorbicillinoid natural products.
-
- Harvey AL; Edrada-Ebel R; Quinn RJ The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov 2015, 14, 111–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

