Building Reversible Nanoraspberries
- PMID: 33600190
- PMCID: PMC8031639
- DOI: 10.1021/acs.nanolett.0c05059
Building Reversible Nanoraspberries
Abstract
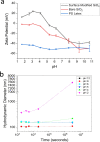
The adsorption mechanism of small positively charged silica nanoparticles (SiO2 NPs) onto larger polystyrene latex nanoparticles (PSL NPs) forming hybrid particles was studied. CryoTEM showed the morphology of these supraparticles to be raspberry-like. After surface modification of the SiO2 NPs, the optimum pH regime to initiate the formation of nanoraspberries was determined. Thereafter, their size evolution was evaluated by dynamic light scattering for different surface charge densities. Reversibility of nanoraspberry formation was shown by cycling the pH of the mixture to make interparticle forces either attractive or repulsive, while their stability was confirmed experimentally. The number of SiO2 NPs on the PSL NPs as determined with cryoTEM matched the theoretically expected maximum number. Understanding and controlling the relevant parameters, such as size and charge of the individual particles and the Debye length, will pave the way to better control of the formation of nanoraspberries and higher-order assemblies thereof.
Keywords: CryoTEM; Raspberry Nanoparticles; Self-assembly; Silica Nanoparticles; Supraparticles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Grzybowski B. A.; Wilmer C. E.; Kim J.; Browne K. P.; Bishop K. J. M. Self-assembly: From crystals to cells. Soft Matter 2009, 5, 1110–1128. 10.1039/b819321p. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

