Eprenetapopt Plus Azacitidine in TP53-Mutated Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Phase II Study by the Groupe Francophone des Myélodysplasies (GFM)
- PMID: 33600210
- PMCID: PMC8099409
- DOI: 10.1200/JCO.20.02342
Eprenetapopt Plus Azacitidine in TP53-Mutated Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Phase II Study by the Groupe Francophone des Myélodysplasies (GFM)
Abstract
Purpose: TP53-mutated (TP53m) myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) have very poor outcome irrespective of the treatment received, including 40% responses (20% complete remission [CR]) with azacitidine (AZA) alone, short response duration, and a median overall survival (OS) of approximately 6 months. Eprenetapopt (APR-246), a novel first-in-class drug, leads to p53 protein reconformation and reactivates its proapoptotic and cell-cycle arrest functions.
Patients and methods: This phase II study assessed the safety and efficacy of eprenetapopt in combination with AZA in untreated high or very high International Prognostic Scoring System-R TP53m MDS and AML patients.
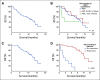
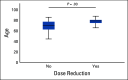
Results: Fifty-two TP53m patients (34 MDS, 18 AML [including seven with more than 30% blasts]) were enrolled. In MDS, we observed an overall response rate (ORR) of 62%, including 47% CR, with a median duration of response at 10.4 months. In AML, the ORR was 33% including 17% CR (27% and 0% CR in AML with less than and more than 30% marrow blasts, respectively). Seventy-three percent of responders achieved TP53 next-generation sequencing negativity (ie, variant allele frequency < 5%). The main treatment-related adverse events were febrile neutropenia (36%) and neurologic adverse events (40%), the latter correlating with a lower glomerular filtration rate at treatment onset (P < .01) and higher age (P = .05), and resolving with temporary drug interruption without recurrence after adequate eprenetapopt dose reduction. With a median follow-up of 9.7 months, median OS was 12.1 months in MDS, and 13.9 and 3.0 months in AML with less than and more than 30% marrow blasts, respectively.
Conclusion: In this very high-risk population of TP53m MDS and AML patients, eprenetapopt combined with AZA was safe and showed potentially higher ORR and CR rate, and longer OS than reported with AZA alone.
Trial registration: ClinicalTrials.gov NCT03588078.
Conflict of interest statement
Figures



Comment in
-
Drugging the Master Regulator TP53 in Cancer: Mission Possible?J Clin Oncol. 2021 May 10;39(14):1595-1597. doi: 10.1200/JCO.21.00192. Epub 2021 Apr 2. J Clin Oncol. 2021. PMID: 33797953 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

