Difficulties in Differentiating Coronaviruses from Subcellular Structures in Human Tissues by Electron Microscopy
- PMID: 33600302
- PMCID: PMC8007326
- DOI: 10.3201/eid2704.204337
Difficulties in Differentiating Coronaviruses from Subcellular Structures in Human Tissues by Electron Microscopy
Abstract
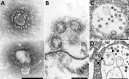
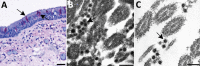
Efforts to combat the coronavirus disease (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have placed a renewed focus on the use of transmission electron microscopy for identifying coronavirus in tissues. In attempts to attribute pathology of COVID-19 patients directly to tissue damage caused by SARS-CoV-2, investigators have inaccurately reported subcellular structures, including coated vesicles, multivesicular bodies, and vesiculating rough endoplasmic reticulum, as coronavirus particles. We describe morphologic features of coronavirus that distinguish it from subcellular structures, including particle size range (60-140 nm), intracellular particle location within membrane-bound vacuoles, and a nucleocapsid appearing in cross section as dense dots (6-12 nm) within the particles. In addition, although the characteristic spikes of coronaviruses may be visible on the virus surface, especially on extracellular particles, they are less evident in thin sections than in negative stain preparations.
Keywords: COVID-19; SARS; SARS-CoV-2; coronavirus; coronavirus disease; electron microscopy; respiratory infections; severe acute respiratory syndrome coronavirus 2; ultrastructure; viruses; zoonoses.
Figures

References
-
- Pesaresi M, Pirani F, Tagliabracci A, Valsecchi M, Procopio AD, Busardò FP, et al. SARS-CoV-2 identification in lungs, heart and kidney specimens by transmission and scanning electron microscopy. Eur Rev Med Pharmacol Sci. 2020;24:5186–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

