Biogeographical venom variation in the Indian spectacled cobra (Naja naja) underscores the pressing need for pan-India efficacious snakebite therapy
- PMID: 33600405
- PMCID: PMC7924803
- DOI: 10.1371/journal.pntd.0009150
Biogeographical venom variation in the Indian spectacled cobra (Naja naja) underscores the pressing need for pan-India efficacious snakebite therapy
Abstract
Background: Snake venom composition is dictated by various ecological and environmental factors, and can exhibit dramatic variation across geographically disparate populations of the same species. This molecular diversity can undermine the efficacy of snakebite treatments, as antivenoms produced against venom from one population may fail to neutralise others. India is the world's snakebite hotspot, with 58,000 fatalities and 140,000 morbidities occurring annually. Spectacled cobra (Naja naja) and Russell's viper (Daboia russelii) are known to cause the majority of these envenomations, in part due to their near country-wide distributions. However, the impact of differing ecologies and environment on their venom compositions has not been comprehensively studied.
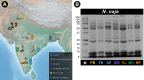
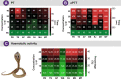
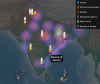
Methods: Here, we used a multi-disciplinary approach consisting of venom proteomics, biochemical and pharmacological analyses, and in vivo research to comparatively analyse N. naja venoms across a broad region (>6000 km; seven populations) covering India's six distinct biogeographical zones.
Findings: By generating the most comprehensive pan-Indian proteomic and toxicity profiles to date, we unveil considerable differences in the composition, pharmacological effects and potencies of geographically-distinct venoms from this species and, through the use of immunological assays and preclinical experiments, demonstrate alarming repercussions on antivenom therapy. We find that commercially-available antivenom fails to effectively neutralise envenomations by the pan-Indian populations of N. naja, including a complete lack of neutralisation against the desert Naja population.
Conclusion: Our findings highlight the significant influence of ecology and environment on snake venom composition and potency, and stress the pressing need to innovate pan-India effective antivenoms to safeguard the lives, limbs and livelihoods of the country's 200,000 annual snakebite victims.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Sunagar K, Casewell N, Varma S, Kolla R, Antunes A, Moran Y. Deadly innovations: unraveling the molecular evolution of animal venoms. Venom Genomics and Proteomics; Springer: Dordrecht, The Netherlands. 2014:1–23.
-
- Whitaker R, Martin G. Diversity and Distribution of Medically Important Snakes of India. In: Gopalakrishnakone P, Faiz A, Fernando R, Gnanathasan CA, Habib AG, Yang CC, editor. Clinical Toxinology in Asia Pacific and Africa. Dordrecht: Springer Netherlands; 2015. p. 115–36.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

