Actionable pharmacogenetic variants in Hong Kong Chinese exome sequencing data and projected prescription impact in the Hong Kong population
- PMID: 33600428
- PMCID: PMC7891783
- DOI: 10.1371/journal.pgen.1009323
Actionable pharmacogenetic variants in Hong Kong Chinese exome sequencing data and projected prescription impact in the Hong Kong population
Abstract
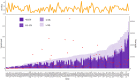
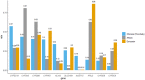
Preemptive pharmacogenetic testing has the potential to improve drug dosing by providing point-of-care patient genotype information. Nonetheless, its implementation in the Chinese population is limited by the lack of population-wide data. In this study, secondary analysis of exome sequencing data was conducted to study pharmacogenomics in 1116 Hong Kong Chinese. We aimed to identify the spectrum of actionable pharmacogenetic variants and rare, predicted deleterious variants that are potentially actionable in Hong Kong Chinese, and to estimate the proportion of dispensed drugs that may potentially benefit from genotype-guided prescription. The projected preemptive pharmacogenetic testing prescription impact was evaluated based on the patient prescription data of the public healthcare system in 2019, serving 7.5 million people. Twenty-nine actionable pharmacogenetic variants/ alleles were identified in our cohort. Nearly all (99.6%) subjects carried at least one actionable pharmacogenetic variant, whereas 93.5% of subjects harbored at least one rare deleterious pharmacogenetic variant. Based on the prescription data in 2019, 13.4% of the Hong Kong population was prescribed with drugs with pharmacogenetic clinical practice guideline recommendations. The total expenditure on actionable drugs was 33,520,000 USD, and it was estimated that 8,219,000 USD (24.5%) worth of drugs were prescribed to patients with an implicated actionable phenotype. Secondary use of exome sequencing data for pharmacogenetic analysis is feasible, and preemptive pharmacogenetic testing has the potential to support prescription decisions in the Hong Kong Chinese population.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- International Conference on Harmonisation; Guidance on E15 Pharmacogenomics Definitions and Sample Coding; Availability. Notice. Fed Regist. 2008;73(68):19074–6. Epub 2008/08/06. . - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

