Effects of storage time and temperature on highly multiparametric flow analysis of peripheral blood samples; implications for clinical trial samples
- PMID: 33600563
- PMCID: PMC7921292
- DOI: 10.1042/BSR20203827
Effects of storage time and temperature on highly multiparametric flow analysis of peripheral blood samples; implications for clinical trial samples
Abstract
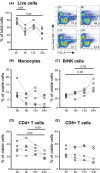
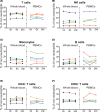
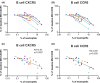
We sought to determine the effect of time and temperature of blood sample storage before preparation of human peripheral blood mononuclear cells (PBMCs) by Ficoll-hypaque density gradient centrifugation. Blood samples from healthy donors were stored at room temperature (RT) or refrigerated at 4°C before preparation of PBMCs. Cell yield and viability, and proportions of major cell populations within PBMCs, as determined by fluorescence flow cytometry, were assessed for both fresh and cryopreserved samples. Highly multiparametric mass cytometry was performed on cryopreserved PBMCs. We found that refrigeration had marked negative effects on subsequent PBMC yield. Storage at RT led to co-purification of low density neutrophils with PBMCs, but had no detectable effects on the proportions of multiple cell subsets including, but not limited to, monocytes, NK cells, B cells, Treg cells, and naïve, central memory and effector memory CD4+ and CD8+ T cells and CD45RA-positive terminal effector CD8+ T cells. Expression of a number of cell surface receptors, including CXCR5, CCR6, CXCR3 and TIGIT, but not CD247 was reduced after RT storage before PBMC preparation, and this effect correlated with the degree of low density neutrophil contamination. As such, when PBMC preparation cannot be undertaken immediately after blood draw, storage at RT is far superior to refrigeration. RT storage leads to neutrophil activation, but does not compromise measurement of PBMC subset distribution. However caution must be applied to interpretation of cytometric measurements of surface molecules such as chemokine receptors.
Keywords: Blood processing; cytometry; immunophenotyping; neutrophil.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures




References
-
- Pirozyan M.R., McGuire H.M., Emran A.A., Tseng H.Y., Tiffen J.C., Lee J.H.et al. (2020) Pretreatment innate cell populations and CD4 T cells in blood are associated with response to immune checkpoint blockade in melanoma patients. Front. Immunol. 11, 372 10.3389/fimmu.2020.00372 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

