Loss of α-actinin-3 during human evolution provides superior cold resilience and muscle heat generation
- PMID: 33600773
- PMCID: PMC8008486
- DOI: 10.1016/j.ajhg.2021.01.013
Loss of α-actinin-3 during human evolution provides superior cold resilience and muscle heat generation
Abstract
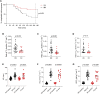
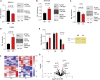
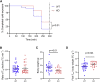
The protein α-actinin-3 expressed in fast-twitch skeletal muscle fiber is absent in 1.5 billion people worldwide due to homozygosity for a nonsense polymorphism in ACTN3 (R577X). The prevalence of the 577X allele increased as modern humans moved to colder climates, suggesting a link between α-actinin-3 deficiency and improved cold tolerance. Here, we show that humans lacking α-actinin-3 (XX) are superior in maintaining core body temperature during cold-water immersion due to changes in skeletal muscle thermogenesis. Muscles of XX individuals displayed a shift toward more slow-twitch isoforms of myosin heavy chain (MyHC) and sarcoplasmic reticulum (SR) proteins, accompanied by altered neuronal muscle activation resulting in increased tone rather than overt shivering. Experiments on Actn3 knockout mice showed no alterations in brown adipose tissue (BAT) properties that could explain the improved cold tolerance in XX individuals. Thus, this study provides a mechanism for the positive selection of the ACTN3 X-allele in cold climates and supports a key thermogenic role of skeletal muscle during cold exposure in humans.
Keywords: alpha-actinin-3 deficincy; brown adipose tissue; energy efficient thermogenesis; evolutionary advantage; improved cold tolerance; muscle fiber type; skeletal muscle.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
V.M.L. is founder, CEO, and shareholder of HepaPredict AB. In addition, V.M.L. discloses consultancy work for EnginZyme AB.
Figures


References
-
- Mills M., Yang N., Weinberger R., Vander Woude D.L., Beggs A.H., Easteal S., North K. Differential expression of the actin-binding proteins, alpha-actinin-2 and -3, in different species: implications for the evolution of functional redundancy. Hum. Mol. Genet. 2001;10:1335–1346. - PubMed
-
- North K.N., Yang N., Wattanasirichaigoon D., Mills M., Easteal S., Beggs A.H. A common nonsense mutation results in alpha-actinin-3 deficiency in the general population. Nat. Genet. 1999;21:353–354. - PubMed
-
- Quinlan K.G., Seto J.T., Turner N., Vandebrouck A., Floetenmeyer M., Macarthur D.G., Raftery J.M., Lek M., Yang N., Parton R.G. Alpha-actinin-3 deficiency results in reduced glycogen phosphorylase activity and altered calcium handling in skeletal muscle. Hum. Mol. Genet. 2010;19:1335–1346. - PubMed
-
- MacArthur D.G., Seto J.T., Raftery J.M., Quinlan K.G., Huttley G.A., Hook J.W., Lemckert F.A., Kee A.J., Edwards M.R., Berman Y. Loss of ACTN3 gene function alters mouse muscle metabolism and shows evidence of positive selection in humans. Nat. Genet. 2007;39:1261–1265. - PubMed
-
- Houweling P.J., Papadimitriou I.D., Seto J.T., Pérez L.M., Coso J.D., North K.N., Lucia A., Eynon N. Is evolutionary loss our gain? The role of ACTN3 p.Arg577Ter (R577X) genotype in athletic performance, ageing, and disease. Hum. Mutat. 2018;39:1774–1787. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

