Antisense technology: A review
- PMID: 33600796
- PMCID: PMC8005817
- DOI: 10.1016/j.jbc.2021.100416
Antisense technology: A review
Abstract
Antisense technology is beginning to deliver on the broad promise of the technology. Ten RNA-targeted drugs including eight single-strand antisense drugs (ASOs) and two double-strand ASOs (siRNAs) have now been approved for commercial use, and the ASOs in phase 2/3 trials are innovative, delivered by multiple routes of administration and focused on both rare and common diseases. In fact, two ASOs are used in cardiovascular outcome studies and several others in very large trials. Interest in the technology continues to grow, and the field has been subject to a significant number of reviews. In this review, we focus on the molecular events that result in the effects observed and use recent clinical results involving several different ASOs to exemplify specific molecular mechanisms and specific issues. We conclude with the prospective on the technology.
Keywords: RNase H1; antisense; clinical results; molecular mechanisms; splicing.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


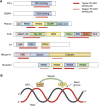
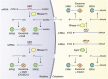

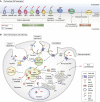
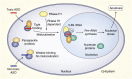
References
-
- Crooke S.T., Witztum J.L., Bennett C.F., Baker B.F. RNA-targeted therapeutics. Cell Metab. 2018;27:714–739. - PubMed
-
- Crooke S.T., Seth P.P., Vickers T.A., Liang X.H. The interaction of phosphorothioate-containing RNA targeted drugs with proteins is a critical determinant of the therapeutic effects of these agents. J. Am. Chem. Soc. 2020;142:14754–14771. - PubMed
-
- Crooke S.T., Baker B.F., Crooke R.M., Liang X.-H. Antisense technology: An overview and prospectus. Nat. Rev. Drug Discov. 2020 In press. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

