Short-Chain Fatty Acids Outpace Ketone Oxidation in the Failing Heart
- PMID: 33601938
- PMCID: PMC8096711
- DOI: 10.1161/CIRCULATIONAHA.120.052671
Short-Chain Fatty Acids Outpace Ketone Oxidation in the Failing Heart
Abstract
Background: The failing heart is energy starved with impaired oxidation of long-chain fatty acids (LCFAs) at the level of reduced CPT1 (carnitine palmitoyltransferase 1) activity at the outer mitochondrial membrane. Recent work shows elevated ketone oxidation in failing hearts as an alternate carbon source for oxidative ATP generation. We hypothesized that another short-chain carbon source, short-chain fatty acids (SCFAs) that bypass carnitine palmitoyltransferase 1, could similarly support energy production in failing hearts.
Methods: Cardiac hypertrophy and dysfunction were induced in rats by transverse-aortic constriction (TAC). Fourteen weeks after TAC or sham operation, isolated hearts were perfused with either the 4 carbon, 13C-labeled ketone (D3-hydroxybutyrate) or the 4 carbon, 13C-labeled SCFA butyrate in the presence of glucose and the LCFA palmitate. Oxidation of ketone and SCFA was compared by in vitro 13C nuclear magnetic resonance spectroscopy, as was the capacity for short-chain carbon sources to compensate for impaired LCFA oxidation in the hypertrophic heart. Adaptive changes in enzyme expression and content for the distinct pathways of ketone and SCFA oxidation were examined in both failing rat and human hearts.
Results: TAC produced pathological hypertrophy and increased the fractional contributions of ketone to acetyl coenzyme-A production in the tricarboxylic acid cycle (0.60±0.02 sham ketone versus 0.70±0.02 TAC ketone; P<0.05). However, butyrate oxidation in failing hearts was 15% greater (0.803±0.020 TAC SCFA) than ketone oxidation. SCFA was also more readily oxidized than ketone in sham hearts by 15% (0.693±0.020 sham SCFA). Despite greater SFCA oxidation, TAC did not change short-chain acyl coenzyme-A dehydrogenase content. However, failing hearts of humans and the rat model both contain significant increases in acyl coenzyme-A synthetase medium-chain 3 enzyme gene expression and protein content. The increased oxidation of SCFA and ketones occurred at the expense of LCFA oxidation, with LCFA contributing less to acetyl coenzyme-A production in failing hearts perfused with SCFA (0.190±0.012 TAC SCFA versus 0.3163±0.0360 TAC ketone).
Conclusions: SCFAs are more readily oxidized than ketones in failing hearts, despite both bypassing reduced CPT1 activity and represent an unexplored carbon source for energy production in failing hearts.
Keywords: cardiac; heart failure; ketones; metabolism; short-chain fatty acid.
Figures


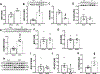
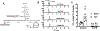
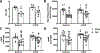
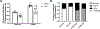
References
-
- Sorokina N, O’Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of Compensatory Pathways to Sustain Oxidative Flux With Reduced Carnitine Palmitoyltransferase I Activity Characterizes Inefficiency in Energy Metabolism in Hypertrophied Hearts. Circulation. 2007;115:2033–2041. - PubMed
-
- Pound KM, Sorokina N, Ballal K, Berkich DA, Fasano M, Lanoue KF, Taegtmeyer H, O’Donnell JM, Lewandowski ED. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ Res. 2009;104:805–812. - PMC - PubMed
-
- Lahey R, Carley AN, Wang X, Glass CE, Accola KD, Silvestry S, O’Donnell JM, Lewandowski ED. Enhanced Redox State and Efficiency of Glucose Oxidation With miR Based Suppression of Maladaptive NADPH-Dependent Malic Enzyme 1 Expression in Hypertrophied Hearts. Circ Res. 2018;122:836–845. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

