Safety and efficacy of Angio-Seal device for transfemoral neuroendovascular procedures: A systematic review and meta-analysis
- PMID: 33601976
- PMCID: PMC8493339
- DOI: 10.1177/1591019921996100
Safety and efficacy of Angio-Seal device for transfemoral neuroendovascular procedures: A systematic review and meta-analysis
Abstract
Introduction: Angio-Seal is a commonly used device for femoral hemostasis in neuroendovascular procedures. This meta-analysis investigates of the safety and efficacy of Angio-Seal in patients undergoing endovascular neurointerventional procedures.
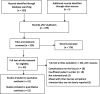
Methods: A systematic review and meta-analysis on all studies evaluating the Angio-Seal device in neurointerventional procedures from inception through 2020 were performed. We studied rates of groin hematoma, retroperitoneal hematoma, pseudoaneurysm, ipsilateral DVT, and ischemic complications. Meta-analysis was performed using the random-effects model.
Results: 13 studies were included in our analysis. 2250 patients with 104 complications were found {4.5% (95% CI, 2.7%-6.3%)}. Of these complications, groin hematoma was the most common with a rate of 2.4% (95% CI, 1.1%-3.6%). Retroperitoneal hematoma {0.3% (95% CI, 0%-0.5%)}, pseudo-aneurysm {0.5% (95% CI, 0.2%-0.8%), and ipsilateral DVT {0.3% (95% CI, 0.1%-0.7%) were also not in negligible rate. The rate of other complications were as follows: vessel occlusion/stenosis; 0.2% (95% CI, 0%-0.4%), vascular surgery; 0.2% (95% CI, 0%-0.5%), and infection; 0.2% (95% CI, 0%-0.5%). One patient died as result of hemorrhagic complications {0.1% (95% CI, 0%-0.3%)}. Use of anticoagulant/antiplatelet therapy was found to be positively correlated with high risk of any groin complication and groin hematoma (p ≤ .05). Female gender was associated with high risk of ipsilateral DVT (p ≤ .05). Interestingly, large sheath size was associated with low risk of groin hematoma (p ≤ .05).
Conclusion: The safety and efficacy rate of Angio-Seal was approximately 95%. The most common complication was groin hematoma. Serious complications including retroperitoneal hematoma and femoral artery occlusion were rare.
Keywords: Angio-Seal; transfemoral access; transradial access; vascular closure devices.
Conflict of interest statement
Figures


References
-
- DerSimonian R, Laird N.Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. - PubMed
-
- The jamovi project. jamovi (Version 1.2) [Computer Software]. https://www.jamovi.org (2020, accessed February 2021)
-
- Wallace BC, Dahabreh IJ, Trikalinos TA, et al. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw 2012; 49: 1–15.
-
- Deeks JJ, Higgins JPT, Altman DG. (eds). Chapter 9: analysing data and undertaking meta-analyses. In: Higgins JPT and Green S (eds) Cochrane handbook for systematic reviews of interventions (Version 5.1.0). London: The Cochrane Collaboration, 2011. pp. 9.33–9.34.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

