Better understanding the neurobiology of primary lateral sclerosis
- PMID: 33602014
- PMCID: PMC8016556
- DOI: 10.1080/21678421.2020.1837175
Better understanding the neurobiology of primary lateral sclerosis
Abstract
Primary lateral sclerosis (PLS) is a rare neurodegenerative disease characterized by progressive degeneration of upper motor neurons (UMNs). Recent studies shed new light onto the cellular events that are particularly important for UMN maintenance including intracellular trafficking, mitochondrial energy homeostasis and lipid metabolism. This review summarizes these advances including the role of Alsin as a gene linked to atypical forms of juvenile PLS, and discusses wider aspects of cellular pathology that have been observed in adult forms of PLS. The review further discusses the prospects of new transgenic upper motor neuron reporter mice, human stem cell-derived UMN cultures, cerebral organoids and non-human primates as future model systems to better understand and ultimately treat PLS.
Keywords: ALS2; Alsin; Betz cell; Golgi apparatus; bioenergetics; corticospinal motor neuron; endosomes; membrane lipids; mitochondria; primary lateral sclerosis; upper motor neuron.
Conflict of interest statement
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Figures
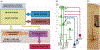


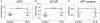

References
-
- Erb WH. Ueber einen wenig bekanten spinalen Symptomenkomplex. About a little-known spinal symptom complex. Berliner Klinische Wochenschrift 1875;26:357–9.
-
- Stark FM, Moersch FP. Primary lateral sclerosis. A distinct clinical entity. J Nerv Mental Dis. 1945;102:332–7.
-
- Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC. Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain 1992;115: 495–520. - PubMed
-
- Gordon PH, Cheng B, Katz IB, Pinto M, Hays AP, Mitsumoto H, et al. The natural history of primary lateral sclerosis. Neurology 2006;66:647–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
